Revista Iberoamericana de Neuropsicología
Vol. 1, No. 1, enero-junio 2018.
Comparison of ICD-10 and DSM-IV Criteria for Postconcussion Syndrome/Disorder
Stephen R. McCauley1,2,3,4, Elisabeth A. Wilde1,2,4,5, Emmy R. Miller6, Claudia S. Robertson7,
James J. McCarthy8,9, and Harvey S. Levin1,2,3,4,7
1 Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston, Texas
2 Department of Neurology, Baylor College of Medicine, Houston, Texas
3 Department of Pediatrics, Baylor College of Medicine, Houston, Texas
4 Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas
5 Department of Pediatric Radiology, Baylor College of Medicine, Houston, Texas
6 Department of Neurosurgery, Virginia Commonwealth University, Richmond, Virginia
7 Department of Neurosurgery, Baylor College of Medicine, Houston, Texas
8 Department of Emergency Medicine, University of Texas Medical School, Houston, Texas
9 Department of Emergency Medicine, Memorial-Hermann Hospital, Houston, Texas
Corresponding author:
Stephen R. McCauley, PhD
Baylor College of Medicine
6501 Fannin St., NB 126,
Houston, Texas 77030
E-mail: mccauley@bcm.edu
voice: 713-798-7479;
fax: 713-798-6898
Comparison of ICD-10 and DSM-IV Criteria for Postconcussion Syndrome/Disorder
Little is known about how the existing diagnostic criteria for postconcussion syndrome/disorder (PCS/PCD) perform in the actual clinical diagnosis of this condition. Both clinical and research evidence to guide diagnosis of PCS/PCD are fraught with inconsistencies. The comparability of studies of patients with PCS/PCD following mild traumatic brain injury (mTBI) is frequently hampered by nonuniformity of symptoms and additional criteria used to diagnose the disorder. This limitation may also contribute to the inconsistency of findings regarding prevalence and outcome following mTBI. Although the International Classification of Diseases 10th Edition (ICD-10) has clinical and research criteria for PCS, and the Diagnostic and Statistical Manual 4th Edition (DSM-IV) included provisional criteria for postconcussion disorder (PCD), few studies appear to employ these criteria sets. Consequently, little is known about how these diagnostic criteria perform and which one, if any, is preferred. Exploring this issue, 101 participants with mTBI (ages 18-50 years) were recruited from consecutive admissions to two Level-1 trauma centers in Houston, Texas with outcome measures including: SF-12, Rivermead Post Concussion Symptoms Questionnaire (RPSQ), Center for Epidemiologic Studies-Depression scale (CES-D), and the Connor-Davidson Resilience Scale (CD-RISC). Measures of attention and memory included the Symbol-Digit Modalities Test, Verbal Selective Reminding Test, and the Brief Visuospatial Memory Test-Revised. Prevalence rates for diagnosing PCS/PCD varied widely among the three criteria sets (ICD-10 clinical, ICD-10 research, DSM-IV) and scheduled study occasions. A comparison of prevalence rates at these time points showed substantial dissimilarity in the percentage of participants meeting criteria for PCS/PCD (e.g., ICD-10 clinical = 60.4%; ICD-10 research = 33.7%, and DSM-IV = 27.7% at one week vs. 30.4%, 20.7%, and 13.0%, respectively at three months). In addition, parallel analyses were conducted in which participants with PCD/PCS were compared to those without the disorder. Those with PCS/PCD reported significantly lower general mental health, higher PCS symptom severity, and higher levels of depressive features using the ICD-10 clinical and DSM-IV criteria. Fewer significant differences were found using the ICD-10 research criteria; none reached significance at one week postinjury. Participants meeting vs. not meeting ICD-10 clinical PCS criteria were compared on attention and memory and no significant differences were found for any measure at any study occasion. Although PCS/PCD prevalence rates varied widely, all three sets appear to identify a subgroup of participants with elevated symptom severity. The ICD-10 research criteria perform differently from the other sets and may be relatively less sensitive in the first week postinjury.
Rates of postconcussion syndrome/postconcussional disorder (PCS/PCD) following civilian mild traumatic brain injury (mTBI) ranges from 14% to over 50% depending on the diagnostic criteria used and the time postinjury when patients are assessed(1-7). Little is known about how the existing diagnostic criteria for PCS perform in actual clinical diagnosis of this difficult and troubling condition. Both clinical and research evidence to guide diagnosis of PCS/PCD are fraught with inconsistencies, as well as the use of additional and often poorly defined criteria for making it. There is little, if any, consensus regarding the specific symptom criteria, the number of symptoms required, or the time frame of symptomatology that should be used to formally diagnose PCS/PCD. To this point, a recent study by Laborey et al.(8) has called for a reassessment of the specificity of symptoms used to define PCS/PCD. There also appears to be disagreement about how long symptoms must persist to make a valid diagnosis of PCS despite the fact that this condition is operationally defined in the DSM-IV(9) criteria as well as in the clinical(10) or research(11) criteria of the International Classification of Diseases, 10th Edition (ICD-10). This lack of diagnostic consensus was highlighted in a recent study by Rose et al.(12) who surveyed physician members of the American College of Sports Medicine (ACSM). Survey respondents were asked about the symptom duration necessary for them before diagnosing PCS which ranged from less than two weeks to more than three months. The minimum number of required symptoms deemed necessary also varied from one to more than four symptoms; 55.9% of respondents reported they required only a single PCS symptom as necessary to make the diagnosis where only 14.6% required three symptoms which, at the very least, would be consistent with both DSM-IV and ICD-10 criteria, assuming corresponding symptoms were required. The ACSM survey highlighted the problem in the U.S. as the U.S. respondents were more likely to require only a single symptom for the PCS diagnosis compared to the non-U.S. respondents. These are just a few examples of the lack of a defined and recognized criteria set that Rose et al., and others(2,3,13,14), have underscored the need for a standardized set of criteria to define PCS because such a set is necessary to increase comparability of research studies and to inform clinical management of patients with mTBI.
Efforts have been made to develop guidance for the type of symptoms and their diagnostic clustering that could be used to guide an accurate and reliable diagnosis of PCS/PCD. Previous studies investigating performance differences in making a PCS/PCD diagnosis between the DSM-IV and ICD-10 clinical criteria have found that both perform similarly at three and six months postinjury (e.g., significant between-group differences in health-related quality of life, depression, anxiety, community integration, etc.), albeit with widely differing prevalence rates(2,3,13,14). These efforts have thus far failed to yield any compelling reasons to favor one criteria set over another. To address this issue, this study sought to extend the results of McCauley et al.(2,3) and Boake et al.(13,14) to explore performance differences between the ICD-10 clinical and research criteria for PCS and the DSM-IV criteria for PCD from subacute (one week) to chronic (six months) postinjury stages. Even though the concept of PCS/PCD has been predicated on the persistence of cognitive, affective, and physical symptoms of mTBI that persist beyond expectations of a typical recovery, this study explored the ICD-10 and DSM-IV PCS/PCD criteria across a range of postinjury time points to better understand the temporal trajectory of performance differences of these diagnostic criteria. Thus, it was hypothesized that participants meeting criteria for PCS/PCD would report 1) higher levels of postconcussion symptoms, 2) lower perceptions of mental and physical health, 3) greater depression severity, and 4) lower sense of psychological resilience than those not meeting PCS/PCD criteria. Using the ICD-10 clinical criteria (that does not require evidence of cognitive dysfunction), it was anticipated that participants with PCS would perform more poorly on measures of attention and/or memory compared to those without PCS.
Participants
A consecutive series of patients was recruited prospectively from the two Level-I trauma centers in the greater Houston, Texas metropolitan area. Inclusion criteria included patients aged 18-50 years at the time of injury who presented to, and were treated and released from, the Emergency Department (ED) less than 24 hours following an injury to the head or extremities. Patients were fluent in either English or Spanish. Specific inclusion criteria for patients with mTBI included a documented/verified head injury, Glasgow Coma Scale (GCS)(15) score of 13-15, loss of consciousness < 30 minutes, posttraumatic amnesia (PTA) < 24 hours, and no trauma-related abnormalities on emergent CT scan. Patients with mTBI were assessed with the Galveston Orientation and Amnesia Test (GOAT)(16) to determine if they were in PTA (GOAT score < 75) at the time of study consent. If so, a legally authorized representative would be approached to provide consent; however, no enrolled subjects met this criterion, and all were enrolled with self-consent. The definition of mTBI used in this study followed the guidelines of the Department of Defense(17) and the American Congress of Rehabilitation Medicine(18). All enrolled participants with mTBI had visible evidence of head trauma associated with multiple mechanisms, in the form of bruising/contusions/abrasions to the head, scalp, or face (84%) and scalp lacerations (44%), of which 54% (24/44) required sutures. Participants were excluded from the study for the following criteria: previous head injury requiring hospitalization (including treatment and discharge from an ED), Abbreviated Injury Scale (AIS)(19) score > 3 for any single body region, significant history of pre-existing serious mental disorders (e.g., psychotic disorder, bipolar disorder, or preinjury PTSD formally diagnosed by psychiatrist/psychologist), Alcohol Use Disorders Identification Test (AUDIT)(20,21) score > 8, Drug Abuse Screening Test (DAST-10)(22-24) score > 3, blood alcohol level >80 mg/dL (or other ED chart documentation of clinical intoxication) at the time of informed consent, left-hand dominant (due to neuroimaging requirements of the larger study), presence of contraindications for magnetic resonance imaging (MRI; e.g., shrapnel, ferrous metal implants, pacemaker, claustrophobia, etc.), or a positive urine pregnancy test.
Study sample
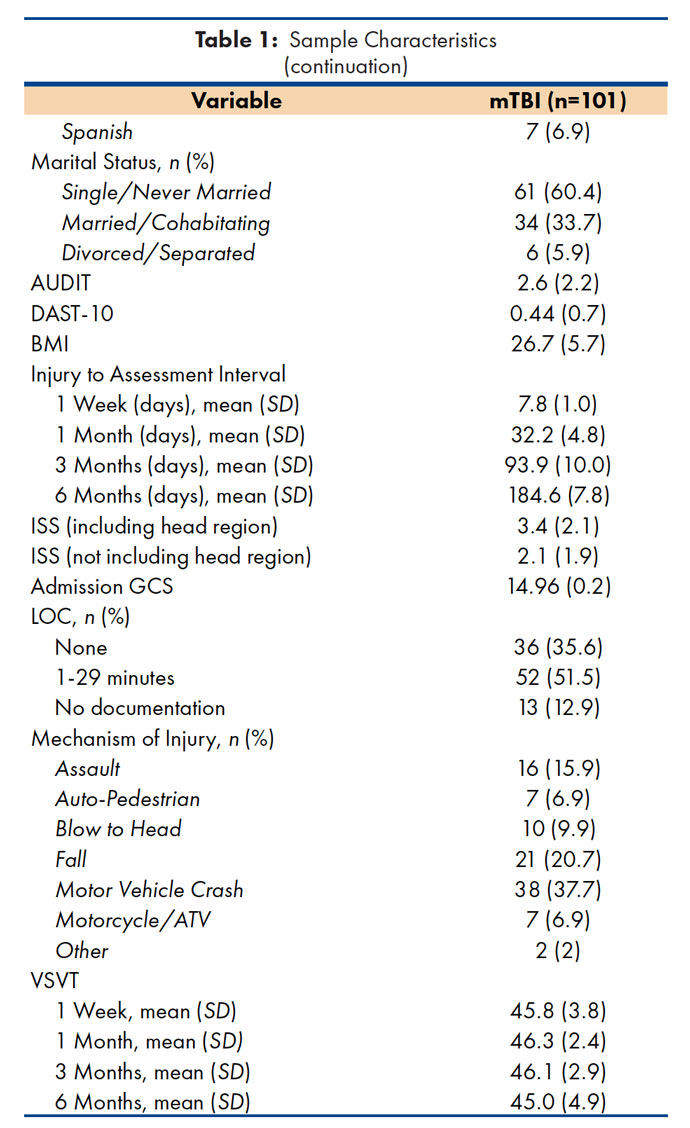
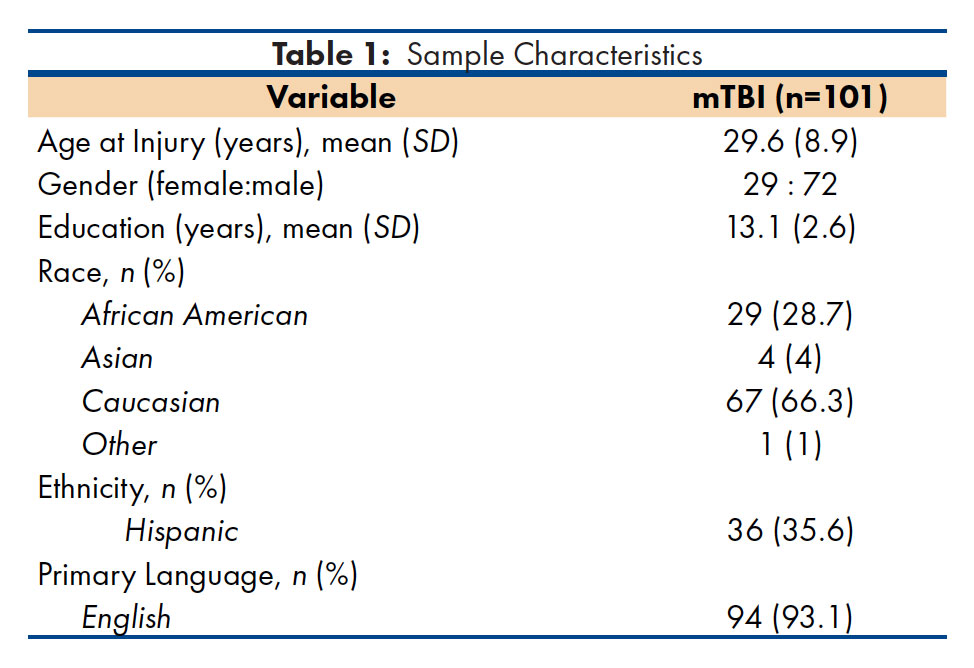
A total of 101 participants with mild TBI were enrolled in the study (see Table 1). As expected, mechanisms of injury in the mTBI group most frequently included motor vehicle crash (MVC; 37.7%) or fall (20.7%). To facilitate inter-study comparisons, the Injury Severity Score (ISS)(19) was reported both with and without the head region as it inflates the ISS for the mTBI group due to coding for concussion.
Measures
Diagnostic and Statistical Manual, 4th Edition (DSMIV): The DSM-IV(9) proposed criteria for PCD include: (A) history of TBI causing “significant cerebral concussion;” (B) cognitive impairment in attention or memory; (C) at least three of eight symptoms (fatigue, sleep disturbance, headache, dizziness, irritability, affective disturbance, personality change, apathy) appearing shortly after injury and persisting for at least 3 months; (D) symptoms beginning after injury or representing a significant worsening of pre-existing symptoms; (E) interference with social and/or occupational functioning; and (F) exclusion of dementia due to head trauma (code 294.1) and other disorders that better account for the reported symptoms. Criteria C and D set a symptom threshold such that symptom onset or worsening must be contiguous to the injury, are distinguishable from pre-existing symptoms, and have a defined minimum duration. DSM-IV criterion A (history of TBI) was determined by the emergency department (ED) trauma physicians, and criterion F (exclusion) was assumed to have been met because of the study’s inclusion/exclusion criteria. Having satisfied criteria A and F, the diagnosis of PCD under DSM-IV was made if the participant’s interview responses satisfied criteria C (symptoms), D (symptom threshold), and E (clinical significance) and if at least one of the participant’s neuropsychological test scores suggested impairment (Criterion B). A neuropsychological impairment of attention or memory was operationally defined as one or more scores of the study’s three measures (two variables each) of attention and memory including: Symbol-Digit Modalities Test (SDMT; written or oral scores), Verbal Selective Reminding Test (VSRT; consistent long-term retrieval and delayed recall), or the Brief Visuospatial Memory Test-Revised (BVMT-R; total recall across three trials and delayed recall) falling 1.5 or more standard deviations away from the mean in the direction of impairment based on published normative data. The DSM-IV criteria were used in this study as the DSM-5 was not yet published when the 5-year study began.
International Classification of Disease, 10th Edition (ICD-10): The ICD-10 includes both clinical and research criteria for PCS. The World Health Organization has published two diagnostic criteria sets for PCS: clinical criteria(10) and research criteria(11). In the notes for users in the research criteria (pages 1-4), the authors state that the research criteria“ …provides specific criteria for the diagnoses contained in Clinical descriptions and diagnostic guidelines.” The ICD-10 clinical criteria require a history of TBI”… usually sufficiently severe to result in loss of consciousness,” three or more of the following eight symptoms must be present (headache, dizziness, fatigue, irritability, insomnia, concentration or memory difficulty, and intolerance of stress, emotion, or alcohol), and the cognitive and other complaints are”… not necessarily associated with compensation motives.” The criteria also state that at least three of the set of required complaints must be present to make a definite diagnosis. Additionally, the guidelines indicate that the symptoms may be accompanied by depression or anxiety resulting from some loss of self-esteem fear of permanent brain damage; these symptoms are not apparently required, however. The diagnosis of PCS under the ICD-10 clinical criteria was made if the participant’s interview responses indicated that three or more of the symptoms listed had been present for at least 1-week postinjury (operationally defined in the absence of a required duration defined by the ICD-10 clinical criteria) and there was no evidence of suboptimal performance on a measure of performance validity. Patients were considered to have met the TBI criterion (determined by the ED trauma physicians) but were not required to have had a documented loss of consciousness (LOC) as the majority of participants in our sample were injured in the absence of a reliable witness and/or were unreliable historians for this information.
The ICD-10 research criteria require history of head trauma resulting in LOC preceding the onset of symptoms by no longer than four weeks. At least three or more of the following 12 symptoms must be present (headache, dizziness, fatigue, noise intolerance, irritability, emotional lability, depression and/or anxiety, subjective complainants of difficulty in concentration and memory problems (without clear objective evidence from psychological tests), insomnia, reduced tolerance to alcohol, and preoccupation with the previous symptoms and fear of permanent brain damage (even hypochondriacal) with the adoption of a sick role. To our knowledge, this criteria set has not previously been used in any published studies. The diagnosis of PCS under the ICD-10 research criteria was made if the participant’s interview responses indicated that three or more of the symptoms listed had been present for at least 1-week postinjury (operationally defined in the absence of a required duration defined by the ICD-10 research criteria). Participants were considered to have met the TBI criterion (determined by the ED trauma physicians).
Connor-Davidson Resilience Scale (CD-RISC): The CD-RISC(25) is a 25-item self-report of psychological resilience with a reported factor structure including constructs of personal competence/tenacity, tolerance of negative affect/stress, positive acceptance of change, internal locus of control, and spirituality. Items are rated using a 5-point Likert scale ranging from 0 – ‘not true at all’ to 4 – ‘true nearly all of the time.’ The total score was used as the primary variable in this study.
Center for Epidemiologic Studies Depression Scale (CES-D): The CES-D(26) is a 20-item self-report measure of depressive symptom severity rated on a 4-point Likert scale (0 – ‘rarely or none of the time’ to 3 – ‘most or all of the time’). Confirmatory factor analysis has demonstrated a factor structure similar to that of the general population (e.g., depressed affect, positive affect, somatic/reduced activity, and interpersonal relationships) in patients with mild to moderate TBI(27). The total score was used as the primary variable in this study.
Rivermead Post Concussion Symptoms Questionnaire (RPCSQ): The RPCSQ(28-30) is a 16-item self-report of cognitive, emotional, and somatic complaints commonly reported following mTBI. Factor analyses have revealed three factors including cognitive, somatic, and emotional problems(30), although differing factor structures also have been reported.(31) The participants rated the severity of each symptom (current experience compared to preinjury levels) from 0 – ‘not experienced at all’ to 4 – ‘severe problem.’ The onset/duration of each symptom was also recorded. The total score was used as the primary variable in this study.
Brief Visuospatial Memory Test-Revised (BVMT-R): The BVMT-R(32) is a measure of visuospatial learning and memory. Participants are shown design stimuli in a 2×3 array for 10 seconds and are asked to draw as many of the figures as they can in the correct location. This procedure is used for three learning trials. The participants are again asked to draw these figures following a 25-minute delay. The primary variables included the total recall across three trials, and the delayed recall score. Scores were standardized using the BVMT-R scoring software and normative data.
Symbol-Digit Modalities Test: The SDMT(33) is a timed, code substitution task that has demonstrated excellent sensitivity in detecting processing speed deficits secondary to cerebral dysfunction assessing the domains of sustained attention and working memory. Using a reference key, the participant is asked to pair numbers associated with simple geometric figures under time constraints. In the written format, the participant writes the correct number below each geometric figure. In the oral format that follows, the participant verbally produces the correct number associated with each figure and the examiner records these responses. The total numbers of correct responses for written and oral versions are the primary variables. Normative data were obtained from the SDMT manual.
SF-12: The 12-item Short Form Survey (SF-12)(34) is a brief measure of physical and psychological well-being that was derived from the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36)(35). It has demonstrated reliability and validity in clinical and population-based studies in the U.S.(34,36) and the short form was found to function as well as the longer SF-36 in longitudinal studies(36). The Mental and Physical Component Summary scores were the primary variables using the normative data provided by the publisher.
Verbal Selective Reminding Test (VSRT): The VSRT(37,38) is a multi-trial measure of verbal learning in which a list of 12 semantically-unrelated words is initially presented. On subsequent trials, only those words not recalled in the previous recall trial are presented until the participant either recalls all 12 words on two consecutive trials, or until all learning trials are exhausted. The six-trial version of the VSRT was used in this study. The primary outcome measures used consistent long-term retrieval (CLTR) and 30-minute delayed recall. Normative data used was that reported by Larrabee, et al.(38) for the six-trial version.
Rationale for Measure Selection
Due to the inclusion of cognitive performance measures required in two of the three criteria sets under study (e.g., DSM-f and ICD-10 research), neuropsychological measures were not appropriate for inclusion as outcome measures. In lieu of this, outcome measures to contrast groups meeting vs. not meeting criteria were selected based on their relevance in assessing outcome following mTBI. Health-related quality of life has been demonstrated to be lower in those with mTBI(6,39-43) and in particular, patients with PCS/PCD(2,3,42). Psychiatric conditions are well-known and documented in mTBI, and depression is a frequently reported postinjury disorder. Although rates differ between studies, depression is a potent pre-injury factor(44-46) and post-mTBI symptom(44,47-50), and depression is often associated with elevated postconcussion symptom severity(51). Although specific postconcussion symptoms are required for diagnosis, the inclusion of a measure of general PCS-like symptoms appeared reasonable, especially as the symptom sets of the DSM-IV and ICD-10 are only partially overlapping with available measures such as the RPCSQ. Psychological resilience is a multi-factorial concept and has been difficult to define precisely; however, it is often conceptualized generally as the ability to maintain a sufficient psychological balance to maintain mental and physical functioning following exposure to aversive stress and/or trauma(52). Components of resilience have been elucidated suggesting that stressors present an opportunity for change/growth(53) and is integral to one’s adaptability to change, strong feelings of self-efficacy, and the formation of secure attachments to others(54) in addition to fostering sufficient tolerance of negative affect(55). In relation to persons with mTBI, McCauley et al.(46) found that preinjury level of resilience was significantly related to trauma-related anxiety and postconcussion symptom severity at one week and one month postinjury; these findings were recently replicated in a similar study by Sullivan et al.(56); resilience was also reported as a key predictor of experiencing PCS-like symptoms following mTBI even after accounting for host factors including age and gender(56). Given these findings, resilience appeared a reasonable attribute to assess as an outcome measure.
Acquisition of PCS/PCD Criteria: Data required to satisfy specific criteria for the diagnosis of PCS/PCD under ICD-10 and DSM-IV were collected in the following manner. Symptoms not included in the RPCSQ (e.g., reduced tolerance to stress, emotional excitement and alcohol, mood swings, etc.) were obtained using the same Likert-scale approach as that of the other RPCSQ symptom items. To consider a symptom as present, the rating for that symptoms had to indicate an increase from before their injury (e.g., a mild, moderate, or severe problem) and were present in the previous week. Structured follow-up questions were used to determine whether or not the symptoms were better accounted for by physical illness, over-the counter medications, illicit substances or alcohol use (DSM-IV criteria), and the level of preoccupation of the participant with her/his physical and/or mental symptoms (ICD-10) was rated as either not present or as mild, moderate, or severe using a standard set of behavioral anchor points. For the cognitive criterion (DSM-IV and ICD-10 research), Z-scores of < -1.5 for total recall and delayed recall of the BVMT-R (linearly transformed from computer-scored T-scores), CLTR and delayed recall of the VSRT, and either the oral or written score of the SDMT were considered indicators of deficits in attention or learning and memory. Only one score indicating impairment was required to satisfy the cognitive impairment criterion. A system of reliability codes ensured that test measures with no modifications or only minor deviations from normal administration were used in the statistical analyses. The investigators acknowledge that the DSM-IV criteria were designed to be used beginning at three months postinjury; however, in the interest of comparing the three criteria sets, this requirement was waived so that performance at each study occasion could be assessed. Procedure
A consecutive series of participants was prospectively screened and recruited from the emergency department (EDs) of the two American College of Surgeons Level-I trauma centers (Ben Taub General Hospital and Memorial Hermann Hospital-Texas Medical Center) in Houston, Texas by study personnel according to a rotating schedule representing all shifts and days of the week. The diagnosis of TBI was made by ED trauma physicians, and Glasgow Coma Scale (GCS) ratings were made by ED trauma physicians or staff. Abbreviated Injury Scale (AIS) ratings were made by AIS-trained research nurses based on thorough medical record review, and results of the AIS body-region severity codes were used to calculate the Injury Severity Score (ISS). All head CT scans were read and coded by a board-certified neuroradiologist.
Participants were administered a baseline assessment (21.8 + 11.5 hours after injury) of their neuropsychological and emotional status. In-person follow-up assessments were also conducted at one week, and at one, three, and six months postinjury by a bachelor’s-level research associate in the participant’s stated preferred language (English or Spanish). Research associates were not blinded to the participant’s injury group status (e.g., mTBI vs. orthopedic controls as part of the larger study). Informed consent was obtained from the participant through an informed consent form and procedure approved by the Institutional Review Boards of Baylor College of Medicine and the University of Texas Medical School-Houston and their affiliate institutions. No participants were recruited while in posttraumatic amnesia (PTA) which would have required consent by a legally authorized representative.
Data analysis
All analyses were conducted using SAS software for Windows, Version 9.4(57). Independent variables were analyzed for outliers; no data points were excluded from the analysis due to extreme scores. Separate analyses were performed at each study occasion (e.g., one week, and one, three, and six months postinjury) using the General Linear Model (GLM) for analysis of variance (ANOVA) for unbalanced data. Bonferroni correction for multiple comparisons was used resulting in an [[α]] < .0008 (.05/60) when comparing groups meeting vs. not meeting criteria for PCS/PCD for the five main outcome measures at each of the four study occasions. Fisher’s Exact Test was used to determine the difference in rates of litigation and receipt of compensation payments between groups.
AUDIT = Alcohol Use Disorders Identification Test.
DAST-10 = Drug Abuse Screening Test, 10-item version.
BMI = Body Mass Index.
ISS = Injury Severity Score.
GCS = Glasgow Coma Scale score.
LOC = Loss of consciousness, medical chart documentation.
ATV = All-terrain vehicle.
VSVT = Victoria Symptom Validity Test, total items raw score.

* Fisher’s Exact Test.
Percentages presented in the table represent the percentages of those either meeting or not meeting PCS/PCD criteria who were involved in litigation or receiving compensation and not the total sample. Results indicate no difference in the rates of involvement in litigation or receiving compensation under any of the three diagnostic criteria sets. Percentages of those involved in litigation in the full sample of participants with mTBI were 17.3%, 10.9%, and 11.3% at 1, 3, and 6 months, respectively.
Percentages of those receiving insurance compensation in the full sample of participants with mTBI were 10.2%, 5.4%, and 5.6% at 1, 3, and 6 months, respectively.
Performance Validity
Effects of suboptimal effort and secondary gain are often reported in the mTBI literature(3,29,58-64) and are required criteria for the ICD-10. Participants were administered the Victoria Symptom Validity Test (VSVT)(65) at each study occasion to verify that optimal effort was obtained during the testing procedure. Effort was evaluated using binomial probability scores (e.g., a total number of correct responses exceeding a criterion indicated valid performance). The VSVT manual states that total scores 30-48 (inclusive) reflect non-suspect effort. All participants produced valid profiles at all time points suggesting that valid and appropriate effort was deployed by these participants toward the neuropsychological testing procedures. Means and standard deviations of the VSVT data are presented in Table 1. To assess the effects of secondary gain, the same methods used by McCauley et al.(3,46,66) were employed; participants were queried regarding their current involvement in litigation or receipt of insurance compensation at one, three, and six months postinjury. The rates of participation in litigation and receiving compensation within those either meeting or not meeting PCS/PCD criteria are presented in Table 2. Overall rates of litigation and compensation involvement for the full sample of participants are given in the footnote of Table 2. Rates of participation in litigation or receipt of compensation did not differ between groups meeting or not meeting PCS/PCD criteria at any study occasion. Previous investigations have consistently found small effect sizes with regard to secondary gain(3,58) and the current data suggest these effects, if present, would be similarly distributed between both groups further allaying any concerns regarding significant biasingeffects.
Prevalence of PCS/PCD
The prevalence rates of those with PCS/PCD were computed for each study occasion, given the wide range of incidence and prevalence rates reported in previous studies. As illustrated in Figure 1, the rates vary widely from 27.7% for DSM-IV to 60.4% for ICD-10 clinical criteria at one week postinjury. Although the prevalence rates predictably declined toward six months postinjury, the same pattern prevails in that the ICD-10 clinical criteria appear the most lenient, the DSM-IV is the most stringent, with the ICD-10 research criteria falling mid-way between the two. This has serious implications for clinical and research uses. To our knowledge, this is the first time that the prevalence rates of PCS/PCD have been compared with both the clinical and research criteria of the ICD-10 and DSM-IV in the same sample of participants with mTBI.
PCS/PCD vs. No-PCS/No-PCD
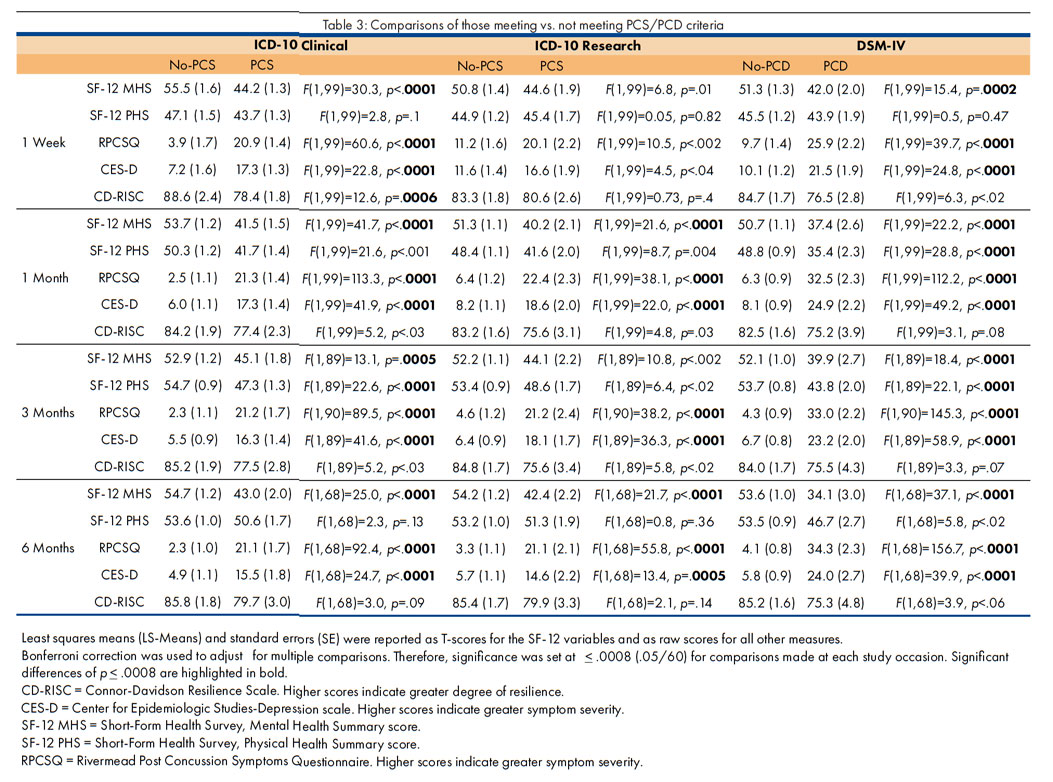
Comparisons were conducted on the main outcome measures (SF-12, RPCSQ, CES-D, CD-RISC) at each study occasion (one week, and one, three, and six months postinjury). Bonferroni correction for multiple comparisons was employed resulting in a stringent [[α]] < .0008 as the criterion for significance (e.g., [[α]] = .05/60 comparisons). ICD-10 Clinical Criteria: Using the ICD-10 clinical criteria (Table 3), significant between-group differences were found for those with vs. without PCS on the SF-12 MHS, RPCSQ, and CES-D at each study occasion (p = .0005 to p < .0001). The SF-12 PHS difference also was significant (p < .0001) at three months postinjury, and a significant difference was found for the CD-RISC at one week postinjury (p = .0006). These findings indicate that the PCS group reported poorer general mental health status, greater postconcussion symptom severity, and higher levels of depressive features compared to those without PCS. At least in the first week postinjury, those in the PCS group perceived lower psychological resilience, but this effect failed to reach significance at later time points.
ICD-10 Research Criteria: Significant between-group differences were found on the SF-12 MHS at one and six months postinjury (p < .0001). Significant differences were also found on the RPCSQ at one, three, and six months (p < .0001), but not at one week postinjury. CES-D comparisons revealed significant differences at one, three, and six months (p = .0005 to p < .0001), but not at one week postinjury. No significant differences were found for the CD-RISC at any study occasion. These findings indicate that the PCS group reported poorer general mental health status, greater postconcussion symptom severity, and higher levels of depressive features compared to those without PCS, but there were no differences in terms of resilience. DSM-IV Criteria: Finally, when using the DSM-IV criteria, significant between-group differences were found for those with vs. without PCD on the SF-12 MHS, RPCSQ, and CES-D at all study occasions (p = .0002 to p < .0001) and the SF-12 PHS differences were significant (p < .0001) at one and three months postinjury. No significant differences were found for the CD-RISC at any study occasion. These findings indicate that the PCD group eported poorer general mental health status, poorer physical health status (at some study occasions), greater postconcussion symptom severity, and higher levels of depressive features compared to those without PCD. The PCD group reported similar levels of psychological resilience compared to those without PCD. PCS and Cognition
The findings from multiple cognitive tests (VSRT, BVMT-R, and SDMT) showed no significant differences between participants meeting PCS criteria and those that did not; furthermore, this was true for all study time points (1 Week, 1 Month, 3 Months, and 6 Months). Both the DSM-IV and ICD-10 research criteria require evidence of cognitive impairment in attention or memory on formal assessment. This obviates the ability to determine meaningful differences in neuropsychological performance between groups with and without PCS/PCD; however, the ICD-10 clinical criteria do not include this criterion. Given this opportunity, analyses were conducted with the six cognitive variables, two each from the SDMT, VSRT, and BVMT-R. Results indicated that no significant differences were found for any cognitive measure at any study occasion. This remained the case even if the criterion for significance was relaxed to a lenient [[α]] < .05 (Table 4).
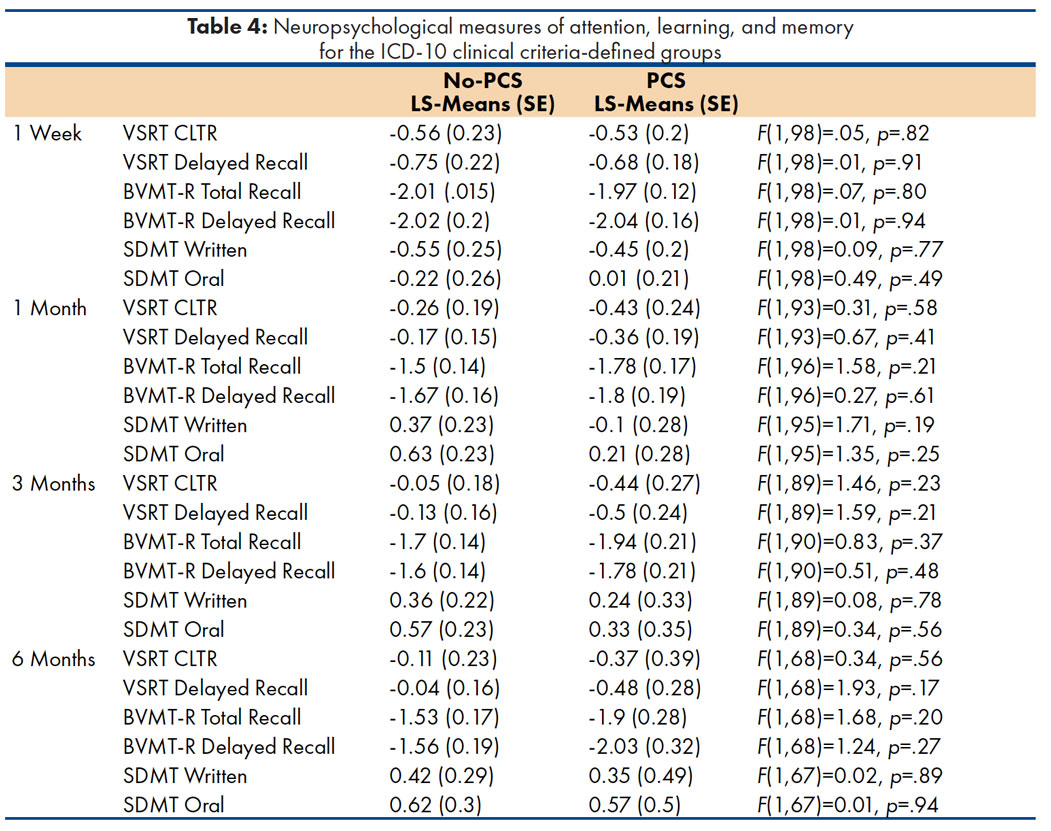
Least squares means (LS-Means) and standard errors (SE) represent Z-scores based on normative data as described in “Measures.”
The T-scores reported using the scoring software for the BVMT-R have been linearly transformed to z-scores to facilitate comparisons with the other cognitive measures.
BVMT-R = Brief Visuospatial Memory Test, Revised.
VSRT = Verbal Selective Reminding Test, six-trial version.
CLTR = Consistent Long-Term Retrieval.
SDMT = Symbol-Digit Modalities Test.
The current study was conducted to understand how three currently established criteria sets to diagnose PCS/PCD perform when compared in the same sample of participants with mTBI. General support was found for the study’s hypotheses in that participants with PCS/PCD were found to report significantly greater postconcussion symptom severity on the RPCSQ. This is not entirely surprising as the required symptoms for the ICD-10 and DSM-IV overlap with the RPCSQ, but the RPCSQ also includes additional unique symptoms and is not wholly redundant with the three criteria sets under study. This strongly suggests that the symptom criteria for DSM-IV and ICD-10 discriminate groups on a wider range of symptom complaints. Similarly, poorer perceptions of general mental health was found in participants with PCS/PCD which is consistent with previous findings(2,3,6,67). Perceptions of physical functioning varied. For ICD-10 clinical, only one significant contrast was found at three months postinjury and the DSM-IV produced differences at one and three months. In contrast, the ICD-10 research criteria found no significant differences at any study occasion. Review of Table 3 suggests that this is due, in part, to the stringent α correction for multiple comparisons; under less strict circumstances, p-values of .004 to < .002. would have been anticipated to be significant, but this suggests some lack of robustness of the criteria set in this area of outcome. Higher levels of depressive symptoms were reported in persons with PCS/PCD at all study occasions using ICD-10 clinical and DSM-IV criteria. This elevated level of depression and depressive symptomatology is consistent with previous findings of mood disorder following mTBI(44,45,47-49,51). In contrast, the ICD-10 research criteria only found significant differences in depression levels beginning at one month postinjury. Although lower preinjury resilience has been associated with higher levels of post-mTBI anxiety and postconcussion symptoms, resilience failed to differ between groups except for the ICD-10 clinical criteria at one week. Given findings of McCauley et al.(46) and Sullivan et al.(56,68), this appeared surprising. It is possible that the stringent [[α]] correction was a contributor to this finding, but a review of the p-values suggests weak differences ranging from p < .02 to nonsignificant trends. Further investigation will be required to better understand the potentially complex interplay of resilience, host factors, and the experience of PCS/PCD which is beyond the scope of this manuscript. In summary and given the level playing field for interpreting these results, the ICD-10 research criteria appear relatively less robust in discriminating PCS vs. No-PCS groups compared to the ICD-10 clinical and DSM-IV. One of the more striking findings from was the wide variations in prevalence rates between the criteria sets. While the DSM-IV and ICD-10 research found comparable rates at one week (27.7% and 33.7%), the ICD-10 clinical resulted in a 60.4% rate of meeting PCS criteria. This has significant implications for research and clinical management of these patients. While clinical lore has posited that between 5-20% of patients develop PCS following mTBI, the rates found when consistently applying the diagnostic criteria far exceeds this commonly held rate. Although the pattern was similar, the rates found in this study differ with those of McCauley et al.(2,3) that included mild and moderate TBI; for instance, at three months postinjury, they found that ICD-10 clinical vs. DSM-IV resulted in 53.8% vs. 17.3% meeting criteria which decreased to 44.6% and 14.4%, respectively, at six months postinjury. The ICD-10 clinical criteria appear to possibly “over-diagnose” PCS compared to the other two criteria sets, particularly at early postinjury time points. One reason for the differences from the McCauley et al. studies and the current investigation is that more severely injured patients were recruited in the earlier studies; however, the pattern between ICD-10 clinical PCS and DSM-IV PCD remains very similar. When reviewing prevalence rates of the ICD-10 research criteria, they appear to fall in the middle ground between the other sets. From an a priori position, it would be difficult to determine what a reasonable prevalence rate for PCS would look like given the lack of consistent defining features that led to the 5-20% rule of thumb estimate in the first place. The ICD-10 and DSM-IV appear to take fairly diametrically opposed positions in terms of psychogenic vs. neurogenic perspectives on postconcussion phenomena. For instance, the DSM-IV requires objective evidence of cognitive impairment in memory or attention (neurogenic perspective) whereas the ICD-10 research requires the patient to report cognitive difficulties in the absence of objective findings of cognitive impairments (e.g., a psychogenic perspective). Consequently, the ICD-10 research criteria produce prevalence rates that fall between the other two. This is attractive in some ways as it avoids what appears to be comparatively extreme rates presented by the other criteria, but the research criteria appear less robust in terms of between-group differences.
This study explored the performance of the ICD-10 clinical criteria in terms of expected cognitive differences detectable in those with and without PCS. The ICD-10 clinical criteria failed to identify any differences in cognition on the most sensitive measures of mTBI currently available even when using the most lenient significance criterion. This result was rather surprising, but it highlights the importance of what the most salient symptoms differentiating those with and without this disorder should include. This underscores the important finding of Laborey et al.(8) that a reassessment of the specificity of symptoms to PCS is needed as they found a common set of eight symptoms drawn from the RPCSQ, DSM-IV, and ICD-10 at three months postinjury were most specific to mTBI compared to controls. Forms of discriminant analyses might present attractive options to better define PCS groups from those with more typical recovery from mTBI, but controversy would remain given the time point at which symptoms constituting “typical recovery” converts to “persistent symptoms” and thus PCS/PCD (e.g., after 4 weeks postinjury, or after 3 months?).
There are some limitations of this study need to be acknowledged. The outcome measures used to characterize the performance of the ICD-10 and DSM-IV criteria sets relied on self-reports. There may be inherent participant-related biases with the results using self-reports as poorer sense of general mental health is likely related to poor mood and greater symptom severity perceptions. Unfortunately, objective measures of cognition to characterize groups with and without PCS/PCD were not possible as cognition was an integral part of defining the groups and one of the required diagnostic criteria. Although other domains could have been assessed to determine the impact of meeting PCS/PCD criteria, other domains besides attention and memory have been shown to be less sensitive to the effects of mTBI which, in turn, limits the usefulness of these domains in such analyses. Since the research staff were not blinded to subject status (e.g., mTBI or orthopedic comparison participant in the larger study), there is always the risk of bias as a threat to internal validity. In this study, this risk was very small, however, as the standardized instruments used present little opportunity for a well-trained, experienced examiner to influence the responses. Conversely, a strength of the current study was the inclusion of performance validity measures (VSVT) and the identification of potential sources of secondary gain and/or malingering. Review of the VSVT data indicates that the participants expended non-suspect levels of effort toward the measures which raises confidence of high data quality and the conclusions drawn from the data. The examination of performance validity should be strongly considered in any study of mTBI given reported effects of secondary compensation and litigation. The rates of involvement in litigation and compensation were characterized, found to be low across study occasions (litigation: 11.3% to 17.3% for the full sample; compensation: 5.4% to 10.2% for the full sample), and the effect of these attributes was distributed similarly between PCS/PCD and No-PCS/No-PCD groups which eliminates concerns regarding the biasing of results or the tendency of one of the criteria sets to be disproportionately affected by these participant attributes.
In conclusion, clinicians and researchers are faced with the challenge of choosing which of these three options as the most appropriate when diagnosing PCS/PCD. It would appear clear that the ICD-10 clinical criteria as outlined is far too lenient a guideline, particularly in the first few weeks postinjury. Eliminating this option, one is faced with selecting either a criteria set that appears to take a predominantly psychogenic approach to the disorder (ICD-10 research) versus a more neurogenic approach (DSM-IV). While there is no doubt that potential psychogenic factors may be involved in recovery from a mTBI for some individuals, evidence is mounting from neuroimaging studies that mTBI results in measurable changes in brain structure and function. This evidence would tend to favor the more neurogenic approach taken by the DSM-IV. Now that the DSM-5 has been published, new challenges have arisen in terms of how to diagnose PCS/PCD. Currently, PCD would most likely be diagnosed as a Mild Neurocognitive Disorder due to traumatic brain injury. It is likely that the DSM-5 criteria will not identify patients with persistent postconcussive symptoms at the same rate as it has eliminated the symptom criterion (DSMIV Criterion C) retaining only objectively measures of cognitive declines (DSM-IV Criterion B). This will likely weaken the utility of the DSM-5 criteria for diagnosing PCD given that Boake et al.(14) found that the cognitive criterion was not specific to TBI compared to those with extracranial injuries; only the combination of Criteria C and D (symptoms and duration) were found to be specific for TBI. Clearly, further study will be required to refine the essential features of this disorder and delineate potential psychogenic and neurogenic factors that result in persistent symptoms presentation following mTBI.
- Meares S, Shores EA, Taylor AJ, Batchelor J, Bryant RA, Baguley IJ, et al.: Mild traumatic brain injury does not predict acute postconcussion syndrome. J Neurol Neurosurg Psychiatry. 2008;79(3):300-6.
- McCauley SR, Boake C, Pedroza C, Brown SA, Levin HS, Goodman HS, et al.: Postconcussional disorder: Are the DSM-IV criteria an improvement over the ICD-10? J Nerv Ment Dis. 2005;193(8):540-50.
- McCauley SR, Boake C, Pedroza C, Brown SA, Levin HS, Goodman HS, et al.: Correlates of persistent postconcussional disorder: DSM-IV criteria versus ICD-10. J Clin Exp Neuropsychol. 2008;30(3):360-79.
- Dean PJ, O’Neill D, Sterr A: Post-concussion syndrome: prevalence after mild traumatic brain injury in comparison with a sample without head injury. Brain Inj. 2012;26(1):14-26.
- Lannsjo M, af Geijerstam JL, Johansson U, Bring J, Borg J: Prevalence and structure of symptoms at 3 months after mild traumatic brain injury in a national cohort. Brain Inj. 2009;23(3):213-9.
- Theadom A, Parag V, Dowell T, McPherson K, Starkey N, Barker-Collo S, et al.: Persistent problems 1 year after mild traumatic brain injury: a longitudinal population study in New Zealand. Br J Gen Pract. 2016;66(642):e16-23.
- Garden N, Sullivan KA: An examination of the base rates of post-concussion symptoms: the influence of demographics and depression. Appl Neuropsychol. 2010;17(1):1-7.
- Laborey M, Masson F, Ribereau-Gayon R, Zongo D, Salmi LR, Lagarde E: Specificity of postconcussion symptoms at 3 months after mild traumatic brain injury: results from a comparative cohort study. J Head Trauma Rehabil. 2014;29(1):E28-36.
- DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association, Inc.; 1994.
- World Health O. The ICD-10 Classification of Mental Disorders and Behavioural Disorders: Clinical descriptions and diagnostic guidelines Geneva: WHO; 1992.
- World Health O. The ICD-10 Classification of Mental Disorders and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: WHO; 1993.
- Rose SC, Fischer AN, Heyer GL: How long is too long? The lack of consensus regarding the post-concussion syndrome diagnosis. Brain Inj. 2015;29(7-8):798-803.
- Boake C, McCauley SR, Levin HS, Contant CF, Song JX, Brown SA, et al.: Limited agreement between criteria-based diagnoses of postconcussional syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(4):493-9.
- Boake C, McCauley SR, Levin HS, Pedroza C, Contant CF, Song JX, et al.: Diagnostic criteria for postconcussional syndrome after mild to moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2005;17(3):350-6.
- Teasdale G, Jennett B.: Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81-4.
- Levin HS, O’Donnell VM, Grossman RG: The Galveston Orientation and Amnesia Test. A practical scale to assess cognition after head injury. Journal of Nervous and Mental Disease. 1979;167(11):675-84.
- Assistant Secretary of Defense for Health Affairs. Health Affairs Memorandum (October 1, 2007). Traumatic Brain Injury: Definition and Reporting. 2007.
- Kay T: Mild traumatic brain injury committee of the head injury interdisciplinary special interest group of the American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86-7.
- Committee on Injury Scaling. Abbreviated Injury Scale. Arlington Heights, IL: Association for the Advancement of Automotive Medicine. 1998.
- Bohn MJ, Babor TF, Kranzler HR: The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. Journal of studies on alcohol. 1995;56(4):423-32.
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M: Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88(6):791-804.
- Skinner HA: The drug abuse screening test. Addict Behav. 1982;7(4):363-71.
- Bohn MJ, Babor TF, Kranzler HR: Validity of the Drug Abuse Screening Test (DAST-10) in inpatient substance abusers. In: Services DoHaH, editor. Proceedings of the 53rd Annual Scientific Meeting, The Committee on Problems of Drug Dependence. Rockville, MD: NIDA Research Monograph. 1991. p. 233.
- Yudko E, Lozhkina O, Fouts A: A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. Journal of substance abuse treatment. 2007;32(2):189-98.
- Connor KM, Davidson JR: Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
- Radloff LS: The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
- McCauley SR, Pedroza C, Brown SA, Boake C, Levin HS, Goodman HS, et al.: Confirmatory factor structure of the Center for Epidemiologic Studies-Depression scale (CES-D) in mild-to-moderate traumatic brain injury. Brain Inj. 2006;20(5):519-27.
- King NS, Crawford S, Wenden FJ, Moss NE, Wade DT: The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587-92.
- Eyres S, Carey A, Gilworth G, Neumann V, Tennant A: Construct validity and reliability of the Rivermead Post-Concussion Symptoms Questionnaire. Clin Rehabil. 2005;19(8):878-87.
- Potter S, Leigh E, Wade D, Fleminger S: The Rivermead Post Concussion Symptoms Questionnaire: a confirmatory factor analysis. J Neurol. 2006;253(12):1603-14.
- Herrmann N, Rapoport MJ, Rajaram RD, Chan F, Kiss A, Ma AK, et al.: Factor analysis of the Rivermead Post-Concussion Symptoms Questionnaire in mild-to-moderate traumatic brain injury patients. J Neuropsychiatry Clin Neu. 2009;21(2):181-8.
- Benedict RH: The Brief Visualspatial Memory Test-Revised(BVMT-R): Manual. Lutz, FL: Psychological Assessment Resources, Inc.; 1995.
- Smith A: Symbol-Digits Modalities Test: Manual. Los Angeles: Western Psychological Services; 1982.
- Ware J, Jr., Kosinski M, Keller SD: A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-33.
- Ware JE, Jr., Sherbourne CD: The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-83.
- Jenkinson C, Layte R, Jenkinson D, Lawrence K, Petersen S, Paice C, et al.: A shorter form health survey: ca the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19(2):179-86.
- Buschke H: Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior. 1973;12:543-50.
- Larrabee GJ, Trahan DE, Levin HS: Normative data for a six-trial administration of the Verbal Selective Reminding Test. Clin Neuropsychol. 2000;14(1):110-8.
- Seidl JN, Pastorek NJ, Lillie R, Rosenblatt A, Troyanskaya M, Miller BI, et al.: Factors related to satisfaction with life in veterans with mild traumatic brain injury. Rehabil Psychol. 2015;60(4):335-43.
- Pieper P, Garvan C: Health-related quality-of-life in the first year following a childhood concussion. Brain Inj. 2014;28(1):105-13.
- Jakola AS, Muller K, Larsen M, Waterloo K, Romner B, Ingebrigtsen T: Five-year outcome after mild head injury: a prospective controlled study. Acta Neurol Scand. 2007;115(6):398-402.
- Ahman S, Saveman BI, Styrke J, Bjornstig U, Stalnacke BM: Long-term follow-up of patients with mild traumatic brain injury: a mixed-method study. J Rehabil Med. 2013;45(8):758-64.
- Emanuelson I, Andersson Holmkvist E, Bjorklund R, Stalhammar D: Quality of life and post-concussion symptoms in adults after mild traumatic brain injury: a population-based study in western Sweden. Acta Neurol Scand. 2003;108(5):332-8.
- Levin HS, McCauley SR, Josic CP, Boake C, Brown SA, Goodman HS, et al.: Predicting depression following mild traumatic brain injury. Arch Gen Psychiatry. 2005;62(5):523-8.
- McCauley SR, Boake C, Levin HS, Contant CF, Song JX: Postconcussional disorder following mild to moderate traumatic brain injury: anxiety, depression, and social support as risk factors and comorbidities. J Clin Exp Neuropsychol. 2001;23(6):792-808.
- McCauley SR, Wilde EA, Miller ER, Frisby ML, Garza HM, Varghese R, et al.: Preinjury resilience and mood as predictors of early outcome following mild traumatic brain injury. J Neurotrauma. 2013;30(8):642-52.
- Levin HS, Brown SA, Song JX, McCauley SR, Boake C, Contant CF, et al.: Depression and posttraumatic stress disorder at three months after mild to moderate traumatic brain injury. J Clin Exp Neuropsychol. 2001;23(6):754-69.
- Vargas G, Rabinowitz A, Meyer J, Arnett PA: Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50(3):250-5.
- Yang J, Peek-Asa C, Covassin T, Torner JC: Post-concussion symptoms of depression and anxiety in division I collegiate athletes. Dev Neuropsychol. 2015;40(1):18-23.
- Schoenhuber R, Gentilini M: Anxiety and depression after mild head injury: a case control study. J Neurol Neurosurg Psychiatry. 1988;51(5):722-4.
- Lange RT, Iverson GL, Rose A: Depression strongly influences postconcussion symptom reporting following mild traumatic brain injury. J Head Trauma Rehabil. 2011;26(2):127-37.
- Bonanno GA: Loss, trauma, and human resilience: have we underestimated the human capacity to thrive after extremely aversive events? The American psychologist. 2004;59(1):20-8.
- Lyons J: Strategies for assessing the potential for positive adjustment following trauma. J Traumatic Stress. 1991;4:93-111.
- Rutter M: Resilience in the face of adversity. Protective factors and resistance to psychiatric disorder. The British journal of psychiatry: the journal of mental science. 1985;147:598-611.
- Kobasa SC: Stressful life events, personality, and health: an inquiry into hardiness. J Personality Soc Psychol. 1979;37:1-11.
- Sullivan KA, Edmed SL, Allan AC, Smith SS, Karlsson LJ: The role of psychological resilience and mTBI as predictors of postconcussional syndrome symptomatology. Rehabil Psychol. 2015;60(2):147-54.
- SAS. Statstical Analysis Software for Windows. Cary, NC: SAS Institute, Inc.; 2012.
- Binder LM, Rohling ML: Money matters: a meta-analytic review of the effects of financial incentives on recovery after closed-head injury. The American journal of psychiatry. 1996;153(1):7-10.
- Paniak C, Reynolds S, Toller-Lobe G, Melnyk A, Nagy J, Schmidt D: A longitudinal study of the relationship between financial compensation and symptoms after treated mild traumatic brain injury. J Clin Exp Neuropsychol. 2002;24(2):187-93.
- Cato MA, Brewster J, Ryan T, Giuliano AJ: Coaching and the ability to simulate mild traumatic brain injury symptoms. Clin Neuropsychol. 2002;16(4):524-35.
- Miller L: Not just malingering: syndrome diagnosis in traumatic brain injury litigation. NeuroRehabilitation. 2001;16(2):109-22.
- Larrabee GJ: Neuropsychological Outcome, Post Concussion Symptoms, and Forensic Considerations in Mild Closed Head Trauma. Semin Clin Neuropsychiatry. 1997;2(3):196-206.
- Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, et al.: Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004(43 Suppl):84-105.
- Hou R, Moss-Morris R, Peveler R, Mogg K, Bradley BP, Belli A: When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83(2):217-23.
- Slick D, Hopp G, Strauss E, Thompson GB: Victoria Symptom Validity Test. Odessa, FL: Psychological Assessment Resources; 1997.
- McCauley SR, Wilde EA, Barnes A, Hanten G, Hunter JV, Levin HS, et al.: Patterns of early emotional and neuropsychological sequelae after mild traumatic brain injury. J Neurotrauma. 2014;31(10):914-25.
- Schiehser DM, Twamley EW, Liu L, Matevosyan A, Filoteo JV, Jak AJ, et al.: The Relationship Between Postconcussive Symptoms and Quality of Life in Veterans With Mild to Moderate Traumatic Brain Injury. J Head Trauma Rehabil. 2015;30(4):E21-8.
- Sullivan KA, Kempe CB, Edmed SL, Bonanno GA: Resilience and Other Possible Outcomes After Mild Traumatic Brain Injury: a Systematic Review. Neuropsychol Rev. 2016;26(2):173-85.
Stephen R. McCauley, PhD, Departments of Physical Medicine and Rehabilitation, Neurology, and Pediatrics,
Baylor College of Medicine; Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas
E-mail: mccauley@bcm.edu; voice: 713-798-7479; fax: 713-798-6898
Elisabeth A. Wilde, PhD, Departments of Physical Medicine and Rehabilitation, Neurology, and Radiology,
Baylor College of Medicine, Houston, Texas; Michael E. DeBakey Veterans Affairs Medical Center,
Houston, Texas
E-mail: ewilde@bcm.edu; voice: 713-798-7331; fax: 713-798-6898
Emmy R. Miller, PhD RN, Department of Neurosurgery, Virginia Commonwealth University, Richmond,
Virginia;
E-mail: emmy.miller@vcuhealth.org; voice: 804 828-6137
Harvey S. Levin, PhD, Departments of Physical Medicine and Rehabilitation, Neurology, Neurosurgery,
and Pediatrics, Baylor College of Medicine, Houston, Texas; Michael E. DeBakey Veterans Affairs Medical
Center, Houston, Texas
E-mail: hlevin@bcm.edu; voice: 713-798-4860; fax: 713-798-6898
Claudia S. Robertson, MD, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas
E-mail: claudiar@bcm.edu; voice: 713-798-4696; fax: 713-873-6609
James J. McCarthy, MD, Department of Emergency Medicine, Memorial-Hermann Hospital and the University
of Texas Medical School, Houston, Texas; UT Health Medical School, Department of Emergency
Medicine, Houston, Texas 77030
E-mail: James.McCarthy50@memorialhermann.org; voice: 713-704-5007; fax: 713-704-5189
Acknowledgment
We sincerely thank the participants for their interest and willingness to take part in this research. This
work was supported by W81XWH-08-2-0131 (McCarthy-PI), W81XWH-08-0132 (Robertson-PI), and
W81XWH-08-2-0133 (Levin-PI) from the Congressionally Directed Medical Research Programs and the
Department of Defense. The information in this manuscript and the manuscript itself has never been
published either electronically or in print. None of the authors have any financial or other relationship(
s) that could be construed as a conflict of interest with respect to the content of this manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official
views of the Department of Defense. The authors also would like to recognize the following persons
for their invaluable assistance in creating Spanish translations, patient recruiting, neuropsychological
assessments, medical data coding, and data entry that made this project possible: Melisa L. Frisby, Hector
M. Garza, Reni Varghese, Carmen Vázquez.