Revista Iberoamericana de Neuropsicología
Vol. 1, No. 2: 163-169, julio-diciembre 2018.
Cognitive Components of Verbal Fluency in non-demented Older Adults with Cerebrovascular Risk Factors. A two-year follow-up
Xóchitl Ortiz-Jimenéz1,2, Fernando Góngora-Rivera1,3, Brenda Saldaña-Muñoz1
1 Center for Research and Development in Health Sciences, Universidad Autónoma de Nuevo León
2 School of Psychology, Universidad Autónoma de Nuevo León
3 School of Medicine, Universidad Autónoma de Nuevo León
Corresponding author:
Xóchitl Angélica Ortiz Jiménez, Ph.D.
Facultad de Psicología
Dr. Canseco 110, Col. Mitras Centro, Monterrey N.L. 64460
Email: xortizj@gmail.com
Cognitive Components of Verbal Fluency in non-demented Older Adults with Cerebrovascular Risk Factors. A two-year follow-up
Objective: To evaluate the semantic and letter verbal fluency performance of older adults with hypertension and/or diabetes (CVR group) and without these risk factors (NR group).
Method: Participants were 60 older adults, 30 from the CVR group and 30 from de NR group, whom completed a neuropsychological assessment at baseline and after two years. Neuropsychological assessment included a semantic verbal fluency (naming animals) and letter verbal fluency task (naming words that begin with letter f). Correct Responses (CR) were analyzed for both fluency tasks, Mean Cluster Size (MC) and Switches (SW) were analyzed for the semantic task, which are measures of Semantic Memory and Controlled Retrieval, respectively.
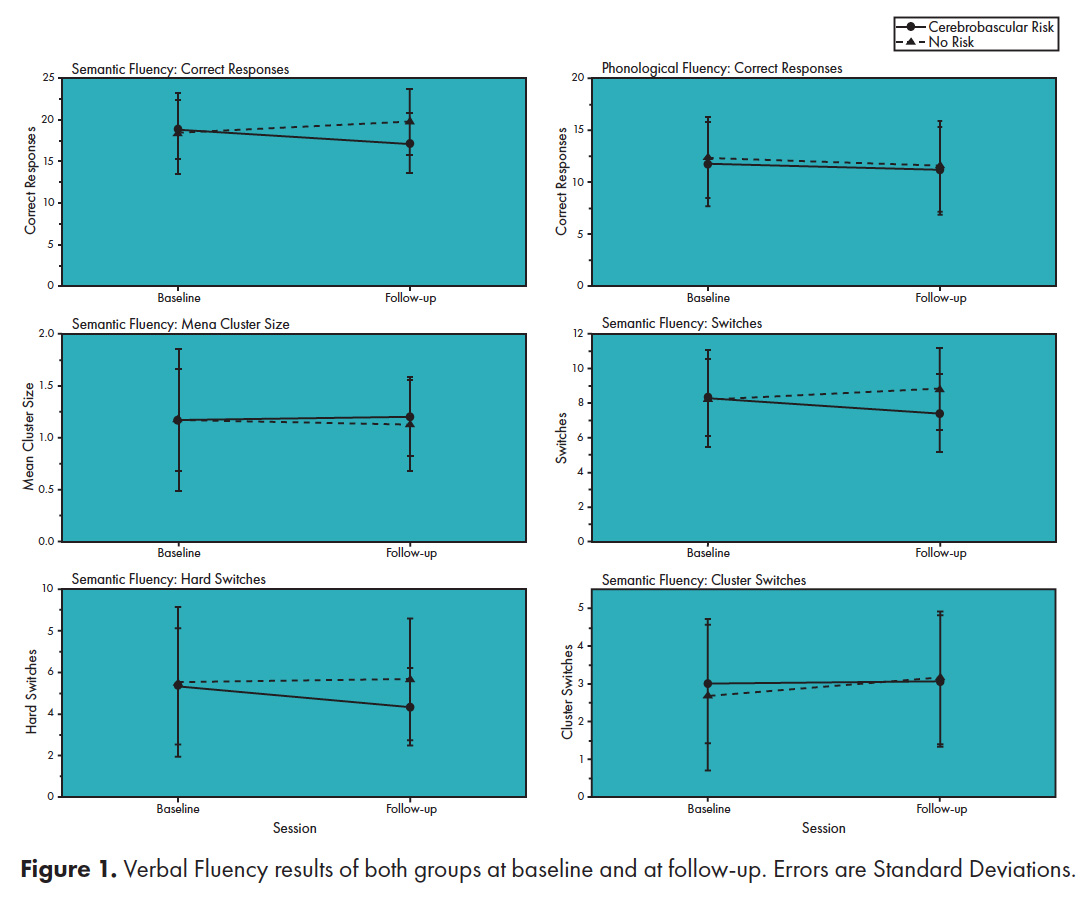
Results: There were no differences between groups at baseline in any measure of both verbal fluency tasks (p>.05). After two years, the CVR group had a lower number of CR and SW (8.3±2.2 vs 7.4±2.3, t (29)= 2.0759, p=.02) on the semantic task while the NR group did not. There was no decline in the letter verbal fluency task of both groups.
Conclusion: Semantic Fluency decline is accelerated by cerebrovascular risk factors, specifically because of a reduction in Controlled Retrieval.
Keywords: Aging, Verbal fluency, Vascular Cognitive Impairment, Hypertension, Diabetes.
Old age is associated to changes in brain structure and function. Subclinical Cerebrovascular disease may be partially responsible for these changes, especially those in the prefrontal cortex, because white matter lesions and neurotransmitter depletion are age-associated changes in anterior brain regions compared with posterior regions, suggesting preferential vulnerability for frontal regions1. These changes could affect cognitive function such as memory and executive functions, leading to what is known as Vascular Cognitive Impairment (VCI), which includes a pre-dementia stage2. The mere presence of cerebrovascular risk factors (CVR), such as hypertension and diabetes, increases the risk for subclinical cerebrovascular disease and accelerates brain and cognitive decline2,3,4. The high prevalence of hypertension and diabetes means that many older adults are at risk of VCI.
Alterations in Prefrontal functioning can lead to memory problems due to poor Controlled Retrieval rather than loss in stored representations5. This makes Retrieval deficits a possible outcome of CVR. One way to dissociate between a deficit in Controlled Retrieval and loss of memory representations is by analyzing verbal fluency performance6.
Verbal Fluency tasks are associated to the left prefrontal and temporal lobe7, and are predictive of problems in daily functioning8. They consist on producing as many words as possible within one minute without any external cues, and involve two neurocognitive components6,9, Prefrontal Controlled Retrieval10 and Semantic Memory. This is because producing words requires the activation of lexical representations in the temporal lobes, and this is achieved through biasing signals from the ventrolateral prefrontal cortex11,12 when there are few cues from the environment. When a lexical representation is activated, automatic activation spreads to other representations that are related to it13, leading to a tendency to produce clusters of related words (farm animals, animals from Africa, aquatic animals) as long as semantic memory is intact. There is less prefrontal involvement for producing clustered words and more when switching between semantic categories10.
Overall performance, Semantic Memory and Controlled Retrieval in verbal fluency tasks are measured with the total number of Correct Responses (CR), Mean Cluster Size (MC) and the total number of Switches (SW), respectively6,9. There are also two types of Switches14, Hard Switches (HS) between a cluster and a non-clustered word, and Cluster Switches (CS) between a cluster and another cluster. The former being a purer measure of Controlled Retrieval, since there is less automatic activation between lexical representations that are not related to each other, requiring more top-down activation from the Prefrontal Cortex for their retrieval10.
Although there is evidence that CVR accelerates decline in verbal fluency’ overall performance in non-demented older adults15, it is not well understood how the underlying neurocognitive mechanisms of verbal fluency are affected. Deficits in Controlled Retrieval and Semantic Memory could lead to different problems in daily functioning and require different compensatory strategies.
Therefore, the objective of this study is to analyze verbal fluency performance and its two neurocognitive components in normal old adults with and without CVR, and to follow-up their performance after two years. In this study, only hypertension and diabetes are taken into account to classify participants as having CVR or not, as the effect of other risk factors on cognitive functioning is less consistent4.
We hypothesize that participants with CVR will have lower verbal fluency performance and that Controlled Retrieval, and not Semantic Memory, will be affected, as measured by CR and SW.
Participants
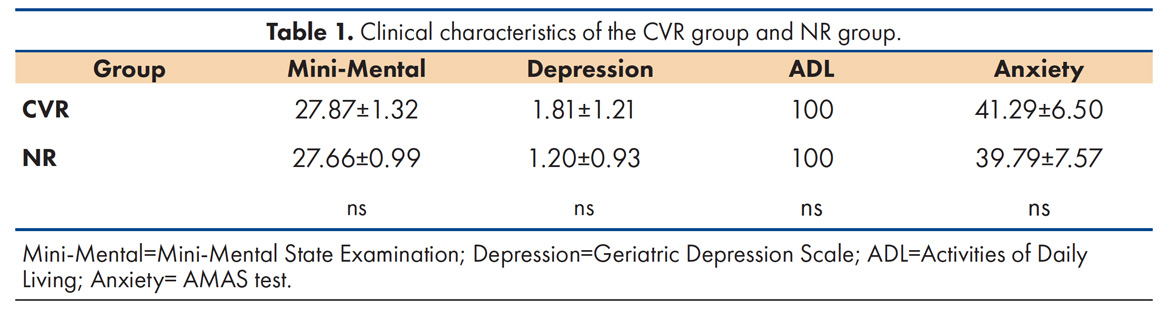
Sixty old adults (38 women) who did not meet the exclusion criteria, such as the presence of dementia and psychiatric disorders like anxiety or depression, were studied in 2014 and had a follow-up in 2016 (see Table 1). Thirty had hypertension and/or diabetes (CVR group) and 30 did not (NR group). The sample was obtained from a larger study, the CIMVAC, which consists of neurological and neuropsychological assessments of retired workers of the Universidad Autonoma de Nuevo Leon and their close relatives of old age. All of them signed an informed consent form. The CVR group (21 women) had a mean age of 69.9±5.7 years and 13.7±4.7 years of education, while the NR group (17 women) had a mean age of 69.3±5.5 years and 15.5±3.9 years of education. There were no significant differences in sex, age, and education between both groups (p>.05). With the exception of one subject in the NR group who had a previous myocardial infarction, none of the participants had any previous heart condition. There were participants with dyslipidemia in both groups, 8 in the NR group and 13 in the CVR group, but this difference is non-significant (p>.05). In the CVR group, 5 participants (25%) reported not taking any medication to treat their hypertension or diabetes at the time of the first session.
Inclusion Criteria
The inclusion criteria were the following: age equal to or greater than 60 years old, have or not have diabetes mellitus type 2, have or not have systemic arterial hypertension, have five or more years of education, acceptance to participate and sign the informed consent.
Exclusion Criteria
The exclusion criteria were the following: having less than five years of education, dementia, depression, anxiety, non-competence in activities of daily living, prior history of stroke, moderate or severe traumatic brain Injury, epilepsy, Parkinson’s disease or other diseases of the central nervous system, and not completing the neuropsychological assessment both at baseline and at the follow up session. The presence of psychiatric disorders and daily functioning was assessed through psychological instruments described below, while neurological conditions were determined by standard physical examination and medical history. The cut-off score of 24 in the MMSE was chosen to categorize participants as having dementia or not, since this has been shown to have good sensitivity and specificity to dementia even without any correction for years of education for people in Mexico16. We excluded participants with less than five years of education because of the effect of this low range of years of education on verbal fluency and MMSE performance, and in order to control for differences in years of education between both groups.
Procedure
The protocol of the study was submitted to the Research Ethics Committee of the Faculty of Medicine of the Universidad Autonoma of Nuevo Leon and was approved with the number NR07-005. Participants completed a neurological and neuropsychological assessment at baseline and at a follow-up session after two years. The neuropsychological assessment was performed by a neuropsychologist or by trained assistants. It consisted of a neuropsychological battery, the Neuropsi Breve, which includes Semantic and Letter Fluency, the Mini-Mental Status Examination, the Geriatric Depression Scale short form, the Adult Manifest Anxiety Scale, and the Instrumental Activities of Daily Living scale. After two years, they were asked for a follow-up session via phone.
Instruments
Semantic verbal fluency and letter verbal fluency consist of naming animals and words with a certain letter for one minute. Letter verbal fluency requires words that start with the letter “F”. We used Troyer9 approach to measure Mean Cluster Size and number of Switches, and Abwender14 approach for measuring Hard Switches and Cluster Switches. We used Troyer9 criteria, with some modifications for the Spanish language and the Mexican population, to determine how words are semantically and phonological related to each other to form clusters. For example, the words “vaca”, “toro” and “buey” are semantically related, and the words “gato” and “pato” are phonological related in Spanish language.
Repetitions and Rule Breaks were not counted as CR and were not taken into account for cluster size, however their position did count in determining switch subtype.
Statistical Analysis
Differences in overall verbal fluency performance at baseline were analyzed with two-tailed t tests for independent groups. The effects of time and the interaction of Time and Group in overall performance were analyzed with a mixed Anova, in case ANOVA indicates that there is interaction, a t test was performed for independent samples at two years. Decline between first and second session in other measures of performance was analyzed with one-tailed t tests for correlated groups, but only when there is decline in overall performance of a fluency task.
There were no differences between groups at baseline in any measure of both verbal fluency tasks (p>.05) (figure 1). There is no effect of time in the number of CR of the Letter task nor in the Semantic Task (p>.05), and there is no interaction between time and group on the number of CR of the Letter task (p>.05), however there is an interaction of time and group on the Semantic task [F (1, 58) = 6.000, p=.017 η² = .094]. The CVR group had a decline in the number of SW (8.3±2.2 vs 7.4±2.3, t (29)= 2.0759, p=.02, d av =.48) and HS (5.3±2.8 vs 4.3±1.9, t(29)=1.8223, p=.04) on the semantic task, compared to their baseline performance, but not in the number of CS and MC (p>.05). The NR group had no decline between their baseline performance and follow-up performance in any of these measures of the semantic task (p>.05). The cognitive components of the letter task were not analyzed, since there was no decline in overall performance.
Decline in overall performance is accelerated by cerebrovascular risk factors on semantic, but not letter fluency. This is the common pattern seen in normal older adults, as only their semantic fluency declines and seems to require compensatory activation of bilateral prefrontal cortex17. Other researchers have found similar results of lower performance or accelerated decline in cognitively healthy people with cerebrovascular risks factors4, 15, although not always18, possibly because of different risk factors in their sample.
Controlled Retrieval, and not Semantic Memory, is the cognitive component of verbal fluency that is affected by cerebrovascular risk. This supports the fact that the structure most affected by cerebrovascular risk is the prefrontal cortex. It also means that memory representations are not being lost but that there is a problem in accessing them due to the accelerated brain loss caused by hypertension and diabetes. This could manifest itself in daily life as a more frequent “tip-of-the-tongue” sensation when speaking.
Decline occurred even though most of the people in the CVR group reported taking proper medication for their hypertension and/or diabetes. A study found worst performance in a prefrontal executive task in participants with hypertension who took their medications compared to participants without hypertension9. Pharmacological treatment of diabetes and hypertension is necessary but might not be sufficient to prevent an accelerated cognitive decline. Cognitive decline might also have the effect of decreasing self-care, making older adults not take their medication properly, which could increase the risk of further cognitive decline and other health problems.
Older adults, especially those with cerebrovascular risk factors, should receive periodic neuropsychological assessment in order to monitor their cognitive health, and cognitive stimulation, such as working memory training, which has been shown to improve cognitive performance and increase prefrontal connections to the posterior cortex20. One problem is that because of the insidious effects of hypertension and diabetes on the brain, and the recurrent belief that cognitive decline is a natural part of life, many older adults don’t seek cognitive-related services until they reach dementia. An effort must be made to educate the public about the risk factors that accelerate cognitive decline, the importance of cognitive health and the steps that can be taken in order to improve it.
The main limitation of the study is the sample size, so the data cannot be generalized and are specific to the sample studied. Another limitation of the study is that we don’t have a third group that only presents hypertension without diabetes, so in future investigations should be included.
Risk factors for cerebrovascular disease, such as hypertension and diabetes, have an effect on cognitive functioning, specifically on the semantic fluency that depends on the prefrontal lobe. The good control and prevention of risk factors can reduce the risk of presenting cognitive deterioration or dementia in older adults.
Financial support
The study was supported by the Faculty of Psychology and the Neurology Service of the UANL.
Acknowledgment
The authors thank the collaborators of the CIMVAC project, specially to Carolina Madrid, Samantha Galván and Gabriela Castro.
Declaration of interests
We declare that we have no financial and personal relationship with other persons, organizations or entities that could influence this article.
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195-208. Available from https://doi.org/10.1016/j.neuron.2004.09.006
- Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. Available from https://doi.org/10.1161/STR.0b013e3182299496
- Debette S, Seshadri S, Beiser A, Au R, Himali J. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461-468. DOI: https://doi.org/10.1212/WNL.0b013e318227b227
- Van Den Berg E, Kloppenborg RP, Kessels RP, Kappelle LJ, Biessels GJ, Jan G. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease. 2009; 1792(5):470-481. Available from: https://doi.org/10.1016/j.bbadis.2008.09.004
- Baldo J, Shimamura A. Frontal lobes and memory. In: Baddeley AD, Kopelman MD, Wilson BA. (Eds). Handbook of Memory Disorders. London: John Wiley & Co; 2003, p. 363-378.
- Troyer A, Moscovitch M, Winocur G, Alexander M. Clustering and switching on verbal fluency: The effects of focal frontal-and temporal-lobe lesions. Neuropsychologia. 1998;36(6):499-504. Available from: https://doi.org/10.1016/S0028-3932(97)00152-8
- Stuss D, Alexander M, Hamer L. The effects of focal anterior and posterior brain lesions on verbal fluency. Journal of the International Neuropsychological Society. 1998;4(3):265-278.
- Burgess PW, Alderman N, Evans J, Emslie H, Wilson BA. The ecological validity of tests of executive function. Journal of the International Neuropsychological Society. 1998;4(6):547–558. Available from: https://doi.org/10.1017/S1355617798466037
- Troyer A, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology. 1997;11(1):138-146.
- Hirshorn EA, Thompson-Schill SL. (2006). Role of the left inferior frontal gyrus in covert word retrieval: Neural correlates of switching during verbal. Neuropsychologia. 2006;44(12):2547–2557. Available from: https://doi.org/10.1016/j.neuropsychologia.2006.03.035
- Martin A. The representation of object concepts in the brain. Annual Review in Psychology. 2007;58:25-45. Available from: https://doi.org/10.1146/annurev.psych.57.102904.190143
- Wagner A, Paré-Blagoev E, Clark J, Poldrack R. Recovering Meaning: Left Prefrontal Cortex Guides Controlled Semantic Retrieval. Neuron. 2001;31(2):329-338.
- Mayr U. (2002). On the dissociation between clustering and switching in verbal fluency: Comment on Troyer, Moscovitch, Winocur, Alexander and Stuss. Neuropsychologia. 2002;40(5):562-566.
- Abwender D, Swan J, Bowerman J. Qualitative analysis of verbal fluency output: Review and comparison of several scoring methods. Assessment. 2001;8(3):323-338. Available from: https://doi.org/10.1177/107319110100800308
- Brady CB, Spiro A, McGlinchey-Berroth R, Milberg W, Gaziano JM. Stroke risk predicts verbal fluency decline in healthy older men: evidence from the normative aging study. Journal of Gerontology B Psychological Science and Social Science. 2011;56(6):340–346. Available from: https://doi.org/10.1093/GERONB/56.6.P340
- Villaseñor-Cabrera T, Guàrdia-Olmos J, Jiménez-Maldonado M, Rizo-Curiel G, Peró-Cebollero M. Sensitivity and specificity of the Mini-Mental State Examination in the Mexican population. Quality & Quantity. 2010;44(6):1105–1112. Available from: https://doi.org/10.1007/s11135-009-9263-6
- Meinzer M, Flaisch T, Wilser L, et al. Neural signatures of semantic and phonemic fluency in young and old adults. Journal of Cognitive Neuroscience. 2009;21(10):2007–2018. Available from: https://doi.org/10.1162/jocn.2009.21219
- Garrett KD, Browndyke JN, Whelihan W, et al. The neuropsychological profile of vascular cognitive impairment—no dementia: comparisons to patients at risk for cerebrovascular disease and vascular dementia. Archives of Clinical Neuropsychology. 2004;19(6):745-757. Available from: https://doi.org/10.1016/j.acn.2003.09.008
- Raz N, Rodrigue KM, Acker JD. Hypertension and the Brain: Vulnerability of the Prefrontal Regions and Executive Functions. Behavioral Neuroscience. 2003;117(6):1169-1180. Available from: https://doi.org/10.1037/0735-7044.117.6.1169
- Metzler-Baddeley C, Foley S, de Santis S, et al. Dynamics of White Matter Plasticity Underlying Working Memory Training: Multimodal Evidence from Diffusion MRI and Relaxometry. Journal of Cognitive Neuroscience. 2017;29(9):1509-1520. Available from: https://doi.org/10.1162/jocn_a_01127