Revista Iberoamericana de Neuropsicología
Vol. 2, No. 1: 43-54, enero-junio 2019.
Development and efficacy of a compensatory skill building program: Parkinson’s disease cognitive rehabilitation for executive functioning (PD-CoRE)
Hannah L. Combs, Ph.D.1, Stella H. Kim, Psy.D.2, & Michele K. York, Ph.D., ABPP-CN1,3
1 Baylor College of Medicine, Department of Neurology, Houston, TX
2 The University of Texas Health Science Center at Houston, Department of Neurology, Houston, TX
3 Michael E. DeBakey Veterans Affairs Medical Center, Parkinson’s disease Research, Education, and Clinical Center (PADRECC), Houston, TX
Corresponding author:
Michele K. York, Ph.D., ABPP-CN
7200 Cambridge St. 9th Floor, Houston, TX 77030
Ph.: 713-798-8673,
fax: 713-798-8573
Email: myork@bcm.edu
Cognitive Components of Verbal Fluency in non-demented Older Adults with Cerebrovascular Risk Factors. A two-year follow-up
Objective: Cognitive dysfunction is a major clinical feature of PD that contributes to disability, caregiver strain, and diminished quality of life over the disease course. Cognitive rehabilitation has mounting evidence as an intervention relevant for improving quality of life for people living with PD. The Parkinson’s Disease Cognitive Rehabilitation of Executive functioning (PD-CoRE) program is a new cognitive rehabilitation program designed to teach compensatory skills that address daily struggles secondary to executive dysfunction and to break the cycle of cognitive impairment, depression, apathy, poor self-efficacy, reduced quality of life, and increased caregiver burden. The aim of the current study was to evaluate the efficacy of the PD-CoRE program in improving executive functions of individuals with PD and mild cognitive impairment.
Methods: Standardized neuropsychological tests and ecologically valid outcome measures were administered to assess executive functions in addition to mood, apathy, self-efficacy, life satisfaction, quality of life, and caregiver burden. A series of Wilcoxon signed-rank tests were performed.
Results: Results revealed initial improvements in immediate attentional capacity and long-term improvements in inhibition, delayed verbal recall, and verbal memory discrimination. 50% of participants reported subjective improvement in their ability to engage in daily activities, and 50% reported increased self-efficacy. Results from informants revealed that 40% of spouses perceived improvements in the participant’s self-regulatory abilities, and 60% reported observing improvements in the participants’ ability to manage activities of daily living.
Conclusion: Findings from the present study provide support for the feasibility and, if cross-validated, the efficacy of the PD-CoRE program in PD patients with executive dysfunction.
Keywords: Parkinson’s disease, executive functions, cognitive rehabilitation, mild cognitive impairment, compensatory strategies, quality of life
Acknowledgments
The authors wish to thank the PD-CoRE group members and their families for their participation, the Houston Area Parkinson Society (HAPS) for their support and assistance with recruitment, and Elizabeth DiNapoli, Brenna Renn, Agustina Rossetti, and Rebecca Martin for their assistance with the development, revisions, and implementation of PD-CoRE.
The hallmark of Parkinson’s disease (PD) is its motor features, but selective cognitive impairments are evident in over 40% of patients (1). Cognitive dysfunction is now recognized as a major clinical feature of PD that contributes more to disability, caregiver strain, and diminished quality of life over the disease course than motor deficits (2-4). Cognitive deficits associated with PD arise in part from neural changes in the frontostriatal circuits, leading to executive dysfunction (5). Thus, difficulties in executive skills such as working memory, inhibition, and cognitive flexibility (or set-shifting) are some of the most prominent and early cognitive changes associated with PD (6-7). With advances in medical interventions for motor symptoms, individuals with PD are living longer and facing greater disability related to cognitive impairments. Unfortunately, pharmacological treatments for PD cognitive changes are currently limited and have not demonstrated efficacy in reducing executive functioning impairments (8). The importance of investigating non-pharmacological treatment options for cognitive dysfunction is clear.
Cognitive rehabilitation is one such behavioral technique with promising evidence to improve and maintain cognitive skills in those with PD-related cognitive impairment. This type of intervention was originally designed for traumatic brain injury but has been adapted for other neurological conditions. However, research is in its infancy and there are no standardized guidelines for treatment in PD. Cognitive rehabilitation programs generally seek to reduce functional impairment and increase engagement in activities of daily living through skills training. Calleo and colleagues (9) conducted a critical integrative review of the PD cognitive rehabilitation literature at the time and found limited evidence for effectiveness across four studies (two of which were randomized controlled trials [RCTs]) 11-12. However, the authors noted numerous potential areas of improvement for future studies, such as the need for ecologically valid outcome measures and generalizability of findings to a larger sample of PD patients. A more recent review (13) found promising benefits for cognitive functioning in mild to moderate PD across seven RCTs, a reflection of both the growing attention to cognitive rehabilitation in this population and the increasing feasibility in this population. Five RCT studies have found statistically significant improvements with moderate effect sizes of cognitive rehabilitation on executive functioning in PD (11, 14-17). While these emerging studies highlight the increasing centrality of non-pharmacological approaches in the care of PD, these findings are preliminary in nature. Participants in these studies tended to be cognitively intact, which limits our understanding of the role of cognitive rehabilitation among those who may benefit the most (i.e., PD patients with mild cognitive impairment). These studies examined a variety of cognitive domains, which limit the generalizability of treatment effects. Executive dysfunction is the most prominent cognitive change in PD, and is more strongly associated with deleterious motor and neuropsychiatric effects in PD (18); therefore, it warrants targeted attention in the development of interventions.
In addition to findings that cognitive rehabilitation may ameliorate cognitive impairment associated with PD, growing attention has been paid to the generalizability of intervention effects to neuropsychiatric symptoms. However, even less research has been conducted in these domains, and the limited findings are inconclusive. Cognitive rehabilitation appears to have a moderate effect on improving depression (11, 14-16, 19), albeit with mixed findings. Even fewer studies have explicitly assessed quality of life, also with mixed findings (11, 16). Apathy, or the reduction in goal-oriented behavior, often overlaps with depression and cognitive impairment but can be evaluated separately (20). As such, apathy is prevalent in PD and associated with poorer quality of life and increased caregiver burden (21-22). Apathy has only been assessed in one RCT(15), with no change noted by authors. Therefore, little is known about potential improvements in apathy following cognitive rehabilitation. A related construct of self-efficacy, or the expectations one has around one’s ability to successfully execute goal-directed behavior(23), has also received limited attention in the PD literature. Self-efficacy is associated with social withdrawal, depression, and diminished functional capacity in patients with Alzheimer’s disease(24). It may operate similarly to the cycle in PD, in which physical deconditioning and cognitive deficits create a loop of helplessness and poor self-efficacy as patients increasingly cease to engage in hobbies, reduce physical activity, rely on others, and feel uncertain about their future. A decrease in self-efficacy may further accelerate physical and cognitive decline. Finally, executive dysfunction and related neuropsychiatric effects of depression, apathy, and poor self-efficacy have deleterious effects on caregiver burden and quality of life(25-27). However, interventions to reduce caregiver burden and improve patient quality of life are scarce. No study to date has assessed all of these interrelated constructs, so it is unknown which of these neuropsychiatric and psychosocial factors are the most amenable to change following participation in a cognitive rehabilitation intervention.
Cognitive rehabilitation has mounting evidence as an intervention relevant for improving quality of life for people living with PD (28-29). Using Calleo and colleagues’ (9) recommendations as a foundation, we developed a novel PD group cognitive rehabilitation program to target the executive functioning impairments in PD, called the Parkinson’s disease Cognitive Rehabilitation of Executive functioning (PD-CoRE) program. We addressed limitations of previous studies by implementing ecologically valid outcome measures in our neuropsychological battery and targeting the intervention to the cognitive deficits most commonly demonstrated in PD patients (i.e., executive functions).
The aim of the current study was to evaluate the efficacy of the PD-CoRE program in building compensatory strategies to improve applied executive functioning in individuals with PD and mild cognitive impairment. Standardized neuropsychological tests and ecologically valid outcome measures were used to assess executive functions in addition to mood, apathy, self-efficacy, life satisfaction, quality of life, and caregiver burden. The goal of PD-CoRE is to teach compensatory skills that address daily struggles secondary to executive dysfunction and to break the cycle of cognitive impairment, depression, apathy, poor self-efficacy, reduced quality of life, and increased caregiver burden. With the skills learned in the PDCoRE program, the following was hypothesized: 1) Participants would perform better on objective measures of executive functioning following the group; 2) Participants would report an improvement in problem-solving and adaptive abilities in their everyday lives, improved mood, increased quality of life, and greater sense of self-efficacy; and 3) Informants would report improved adaptive and self-regulatory abilities.
Study 1.
Initial Feasibility Study
Methods
Participants
A preliminary feasibility study was implemented in January 2016 in which PD-CoRE was delivered in a small group setting. The PD-CoRE program was created to be delivered via groups instead of individual sessions as groups are traditionally more cost-effective than individual sessions (e.g., less time intensive for therapists, require fewer resources; 30). Nine participants (67% male, Agemean = 66.9, Educationmean = 16.22 years), with mild PD (baseline Montreal Cognitive Assessment [MoCAmean] = 25.7/30) and self-reported executive dysfunction were successfully recruited and retained for the initial feasibility group.
Process
The Baylor College of Medicine Institutional Review Board approved the study protocol and informed consent was obtained from all participants. The initial program consisted of 8 1.5-hour group sessions providing education and hands-on experiences targeting inhibition, working memory, and set-shifting abilities. Participants were recruited via flyers distributed in the corresponding author’s clinic and via a posting in the Houston Area Parkinson Society (HAPS) newsletter. Potential subjects who expressed interest were contacted by a group leader and completed an eligibility screener to gather basic demographics (age, sex, race, handedness, and education) and to complete a baseline MoCA, Beck Depression Inventory, 2nd Edition (BDI-II), Columbia- Suicide Severity Scale (CSSR-S), and Parkinson’s Daily Activities Questionnaire-15 (PDAQ-15). Participants were included if they had a diagnosis of idiopathic PD, were between the ages of 45 and 75 years old, were fluent English speakers, and were able to give informed consent. Exclusionary criteria included: active psychosis, significant depression (BDI-II > 14), impaired instrumental activities of daily living (PDAQ-15 < 60), concurrent cognitive rehabilitation treatment, and diagnosis of dementia. All participants completed at least 7 of the 8 weekly sessions. Participants underwent comprehensive neuropsychological batteries prior to the start of group and immediately following the completion of the group. Materials/instruments
Participants completed standardized measures pre- and post-treatment assessing global mental status (MoCA; 31) and memory (Hopkins Verbal Learning Task- Revised [HVLT-R]; 32), as well as multiple aspects of executive functioning including working memory (Digit Span; 33), verbal fluency (Delis-Kaplan Executive Functioning System [D-KEFS] Letter Fluency & Category Fluency; 34), semantic set-shifting (D-KEFS Category Switching, 34), visual scanning/tracking (Trail Making Test Part A, TMT A; 35), psychomotor set-shifting (Trail Making Test Part B, TMT B; 35), and inhibition (Stroop Color Word Test; 36). Participants also completed self-report measures of perceived cognition (Everyday Problems Test, EPT; 37), perceived executive dysfunction (Dysexecutive Questionnaire, DEX; 38), depression (Beck Depression Inventory, 2nd edition, BDI-II; 39), anxiety (Generalized Anxiety Disorder, 7-item scale, GAD-7; 40), impact of PD on functioning and well-being (The Parkinson’s Disease Questionnaire, PDQ-39; 41) and a patient satisfaction questionnaire.
Statistical analysis
All neurocognitive measures were corrected based on appropriate normative data and converted to a common metric (i.e., T Score with M = 50, SD = 10). Examination of the data showed departure from normal distribution for most dependent variables, therefore nonparametric analyses were utilized. All analyses were run using SPSS (Version 24.0, IBM Corp., Armonk, NY). Wilcoxon signed-rank tests (nonparametric paired samples tests) were utilized to evaluate differences between testing prior to the start of PD-CoRE and immediately following the end of PD-CoRE treatment. Alpha was set at 0.10, one-tailed, for all inferential tests.
Preliminary analyses revealed an improvement in set-shifting ability (TMT B; t(8) = 2.14, p = 0.06, d = 0.73; MT1 = 43.89, SDT1 =13.10, MDT2 =49.56, SDT2 =15.89) and a decline in verbal learning (HVLT-R Total; t(8) = 3.45, p = 0.009, d = 1.20; MT1 = 50.00, SDT1 = 7.91, MDT2 = 42.44, SDT2 = 9.77). No other changes were noted on the outcome measures. Examination of mood variables revealed subclinical depressive symptoms at baseline and did not change following treatment (t(8) = -1.769, p = 0.115). Acceptability and satisfaction with the program was high on self-report evaluations; 100% of participants reported enjoying the social interaction with other PD patients, 89% agreed that the program provided them with ecologically valid skills for use in daily life, 78% reported improved problem-solving skills, and 89% would recommend it to other PD patients.
Study 2. PD-CoRE Efficacy Study
Based on participant feedback and expert consensus, the structure and content of the PD-CoRE program was modified to include additional interactive activities and decrease the number of sessions. The expert consensus panel consisted of a Neuropsychologist, a Movement Disorders Neurologist, a Psychiatrist, a community Social Worker from the local Parkinson’s community group, and an individual with Parkinson’s disease. The consensus panel met on two occasions to discuss the development and revision of the manualized treatment program. The revised PD-CoRE format consisted of 6 weekly 1.5-hour group sessions. In the current study, a range of neuropsychiatric and psychosocial variables were assessed at three time-points (pregroup, post-group, and 3 months post-group) to extend preliminary findings identified by Leung and colleagues’ (13) review. Spouse informants were also included to provide collateral ratings. In addition, more stringent inclusion/exclusion criteria were adopted to better assess intervention effects, including selectively targeting those with PD-related mild cognitive impairment (MCI; based on PD-MCI diagnostic criteria published by the Movement Disorder Society Task Force; 42) who may stand to benefit the most from cognitive rehabilitation relative to those with intact abilities. The specific eligibility criteria are discussed below.
Participants
Six patients with PD (50% male, Agemean = 68.3, Educationmean = 15 years) were recruited along with six spouse informants via flyers distributed in the corresponding author’s clinic and via a posting in the Houston Area Parkinson Society (HAPS) newsletter. Potential subjects who expressed interest were contacted by one of the group leaders and completed an eligibility screener to gather basic demographics (age, sex, race, handedness, and education) and to complete a baseline MoCA, BDI-II, CSSR-S, and PDAQ-15. Participants were included if they had a diagnosis of idiopathic PD, were between the ages of 45 and 75 years old, were fluent English speakers, were able to give informed consent, and had MoCA scores between 21 and 25 with > 3 items on delayed recall (43). Exclusionary criteria included: active psychosis, significant depression (BDI-II > 14), impaired instrumental activities of daily living (PDAQ-15 < 60), concurrent cognitive rehabilitation treatment, and diagnosis of dementia. Process
All group members underwent a comprehensive neuropsychological battery prior to the start of the PD-CoRE program that included the original measures from the feasibility study (described above) as well as the Iowa Gambling Task (IGT; 44; measure of decision-making), Satisfaction with Life Scale (SLS; Diener, 45), Generalized Self-Efficacy Scale (SES; 46), Apathy Evaluation Scale (AES; 20), the self-report form of the Penn Parkinson’s Daily Activities Questionnaire-15 (PDAQ; 47; assessment of cognitive instrumental activities of daily living in PD), and the self-report Behavior Rating Inventory of Executive Functions, Adult Version (BRIEF-A; 48). Informants completed the Zarit Burden Scale (49), the informant version of the PDAQ, and the informant version of the BRIEF-A. Following the final group session, all group members underwent a repeat comprehensive neuropsychological battery with the above measures. Three months after the group ended, subjects returned to complete a second repeat neuropsychological evaluation.
Statistical analysis
Similar to the feasibility study, all neurocognitive measures were corrected based on appropriate normative data and converted to a common metric (i.e., T Score with M = 50, SD = 10). Wilcoxon signed-rank tests (nonparametric paired samples tests) were run to evaluate differences before and after the PD-CoRE treatment as well as before treatment and during the 3-month follow-up evaluation. Reliable Change Indices (50) were calculated based on information provided in the original test manuals to evaluate significant change over time for each participant. Given the small sample size, alpha was set at 0.10, one-tailed, for all inferential tests.
Cognitive outcomes
Comparison of neuropsychological performance at various time points (pre-group, post-group, and follow-up) revealed variable interim changes. As compared to their pre-group performance, participants demonstrated improvement (i.e., higher scores) on immediate attentional capacity (LDSF, z = -1.643, p = 0.10; MT1 = 44.83, SDT1 = 7.41, MDT2 = 49.50, SDT2 = 7.45) post-group. Participants demonstrated decline (i.e., lower scores) on measures of speeded word reading (Stroop Word Reading, z = -2.626, p = 0.009; MT1 = 32.50, SDT1 = 11.20, MDT2 = 27.67, SDT2 = 10.09), initial verbal learning (HVLT-R Total Recall; z = -3.081, p = 0.002; MT1 = 54.17, SDT1 = 6.08, MDT2 = 41.67, SDT2 = 9.48), and delayed verbal recall (HVLT-R Delay Recall, z = -1.886, p = 0.059; MT1 = 51.33, SDT1 = 6.68, MDT2 = 43.83, SDT2 = 13.47). Results from the three-month follow-up testing revealed improved inhibition (Stroop Color-Word, z = -1.633, p = 0.10; MT1 = 40.17, SDT1 = 14.80, MT3 = 34.50, SDT3= 12.12), delayed verbal recall (HVLT-R Delay, z = -1.826, p = 0.068; MT1 = 51.33, SDT1 = 6.68, MT3 = 42.75, SDT3= 14.52), and verbal memory discrimination (HVLT-R Discrimination, z = -1.604, p = 0.10; MT1 = 48.00, SDT1 = 4.52, MT3 = 37.50, SDT3 = 11.85) as compared to baseline performance. There were no significant changes between the postgroup evaluation and the three-month follow-up.
Affective/behavioral outcomes
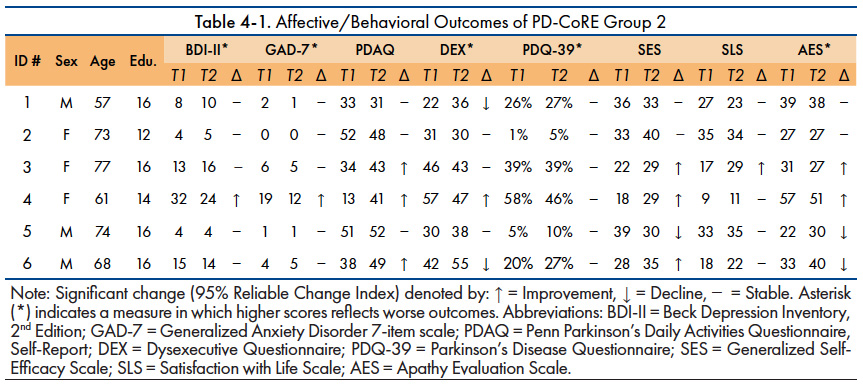
Comparison of affective and behavioral measures at various time points (pre-group, post-group, and follow-up) on a group level revealed no significant differences. However, given the heterogeneity of patient presentation at group onset, analysis of the overall group may mask within participant changes. Table 4-1 provides patient level specifics on various affective/behavioral outcome measures. 50% of participants reported perceived improvement in their ability to engage in daily activities (as measured by the PDAQ) and 50% reported increased self-efficacy.
Informant report
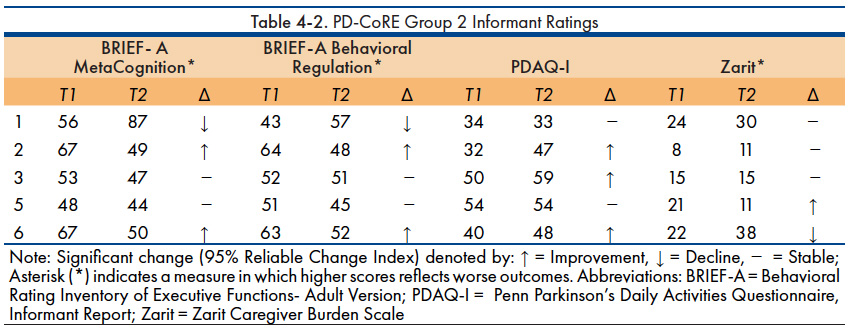
Comparison of informant ratings on a group level revealed no significant changes. Table 4-2 depicts the individual changes in participants as rated by the spouse informants. 40% of the spouses reported improvements in the participant’s self-regulatory abilities, specifically with their ability to regulate thoughts and cognitions (i.e., metacognition) and their ability to regulate behaviors and emotions (i.e., behavioral regulation). 60% of spouses reported observing improvements in the participants’ ability to manage activities of daily living.
Patient satisfaction
All participants completed a post-group satisfaction survey. 100% of the group participants reported that they could apply the skills from the program to their daily life, they reported improved self-confidence and sense of self-efficacy, and also reported enjoying the social interaction with other PD patients. 100% of the participants indicated strong satisfaction with the program, interest in participating in similar programs in the future, and would recommend the program to other Parkinson’s disease patients. Participants commented on the changes they noticed after participating in the group. Responses included “I don’t feel as overwhelmed by trying to do a difficult task, now I can break it down,” and “[I am] more deliberative, better organized, engaged, and more confident.”
Cognitive dysfunction is a major clinical feature of PD that contributes more to disability, caregiver strain, and diminished quality of life over the disease course than motor deficits (2-4). Unfortunately, pharmacological treatments for PD-related cognitive changes are limited and have not demonstrated efficacy in reducing executive functioning impairments (8). As such, it is important to investigate non-pharmacologic treatment options for cognitive dysfunction. The present study evaluated the feasibility and efficacy of a novel cognitive rehabilitation program (PD-CoRE) in improving executive functions in patients with PD. Results from the initial feasibility study suggested that patients with PD enjoyed the program, believed the program provided them with ecologically valid skills for use in daily life, and improved their problem-solving skills. The small group setting also provided immediate emotional support and created a richer learning environment where participants were able to serve as role models for each other, learn from each other’s experiences, and share resources.
Though the participants reported high satisfaction with the program, results from the neuropsychological measures were mixed. The PD-CoRE treatment did not have a significant impact on levels of depression or anxiety. As compared to their baseline performance, participants demonstrated slight improvement in immediate attentional capacity following the PD-CoRE treatment but demonstrated subtle declines on speeded word reading, verbal learning, and delayed verbal recall. Results from the three-month follow-up testing revealed slight improvement in inhibition, delayed verbal recall, and verbal recognition discrimination. The variable and subtle changes on neuropsychological outcome measures was, in the end, unsurprising. The PD-CoRE program focuses on building compensatory strategies to improve applied executive functioning in daily life and is not a form of cognitive training, therefore it does not attempt to improve the underlying cognitive ability. A common issue within neuropsychology is the poor convergence between performance on executive functioning measures (unpracticed ability to execute cognitive processes) and application of executive functioning abilities within the context of real-life situations. As such, capturing changes within a person’s ability to successfully use executive abilities day-to-day is difficult and often relies on patient or informant self-report. In order to address this issue in the current study, we included both patient and informant report.
A strength of the current study is the inclusion of quality of life, self-efficacy, and daily functioning measures in combination with informant ratings and objective neuropsychological data. Biundo and colleagues (51) highlight the disconnect between definitions of “successful” outcomes within the cognitive rehabilitation literature. Empirical research often considers an intervention successful if patients demonstrate improved performance on traditional neuropsychological measures; however, patients and families expect functional improvements or at least functional stability when considering whether a treatment was “successful.” In the current study, 50% of participants self-reported improvement in daily functioning and 50% reported increased self-efficacy. Similarly, more than half of the informants reported noticing improvements in their spouse’s ability to function on a daily basis and 40% reported noticing improved self-regulation.
There are several limitations in the present study that must be acknowledged. First, the sample was quite small and a larger sample would have increased the power to detect treatment effects. The small sample size also precluded more detailed analyses of relationships between variables. Second, participants in the feasibility study tended to be cognitively intact with self-report of mild executive functioning changes; therefore, ceiling effects may have limited detection of intervention effects. Third, the clinical characteristics of the patients in the efficacy study varied widely within the group. In fact, heterogeneity of patients is often considered one of the main critiques of the cognitive rehabilitation for PD literature as the diversity of samples makes it difficult to understand the long-term effectiveness of the treatments (51).
Another limitation of the present study is that changes associated with the group (both positive and negative) were presumed to be related to the group treatment. However, there were participants who reported acute stressors that were unrelated to PD and may have impacted self-report responses. These acute stressors may have impacted the participant’s receptiveness to group teachings. Similarly, information on participant’s medication regimen was not gathered and it is unclear whether any change to the regimen occurred during the course of treatment. As such, it is unknown whether the patient’s pharmacological treatment impacted the cognitive and emotional outcomes.
It is also important to note that the measurement of treatment efficacy is limited to only the functions assessed within the current neurocognitive test battery. Future studies should include additional informant ratings (including informants’ report of program satisfaction) and additional measures of self-regulation. An additional limitation is the lack of a non-treatment PD control group, which would be useful in further investigating the efficacy of the PD-CoRE program.
Overall, the present study’s findings provide support for the feasibility and, if cross-validated, the efficacy of the PD-CoRE program in PD patients with self-reported executive dysfunction. Clinically, professionals working with PD should be aware of potential executive function deficits and potential obstacles that might arise from such impairments within patients’ daily lives as well as the possibility of improvement in executive functioning with appropriate compensatory skill building. By providing PD patients with psychoeducational information and ecologically valid compensatory strategies regarding executive functioning, PD-CoRE may be able to break the negative cycle of PD by improving mood, self-efficacy, and quality of life and reducing caregiver burden.
- Monastero R, Di Fiore P, Ventimiglia GD, Ventimiglia CC, Battaglini I, Camarda R, Camarda C. Prevalence and profile of mild cognitive impairment in Parkinson’s disease. Neurodegenerative diseases. 2012;10(1-4):187-90.
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Movement disorders. 2008 Apr 30;23(6):837-44.
- Lawson RA, Yarnall AJ, Duncan GW, Khoo TK, Breen DP, Barker RA, Collerton D, Taylor JP, Burn DJ. Severity of mild cognitive impairment in early Parkinson’s disease contributes to poorer quality of life. Parkinsonism & related disorders. 2014 Oct 1;20(10):1071-5.
- Leroi I, Harbishettar V, Andrews M, McDonald K, Byrne EJ, Burns A. Carer burden in apathy and impulse control disorders in Parkinson’s disease. International journal of geriatric psychiatry. 2012 Feb;27(2):160-6.
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. Journal of Neuroscience. 2003 Jul 16;23(15):6351-6.
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. The Lancet Neurology. 2010 Dec 1;9(12):1200-13.
- Robbins TW, Cools R. Cognitive deficits in Parkinson’s disease: a cognitive neuroscience perspective. Movement Disorders. 2014 Apr 15;29(5):597-607.
- Vale S. Current management of the cognitive dysfunction in parkinson’s disease: how far have we come?. Experimental biology and medicine. 2008 Aug;233(8):941-51.
- Calleo J, Burrows C, Levin H, Marsh L, Lai E, York MK. Cognitive rehabilitation for executive dysfunction in Parkinson’s disease: application and current directions. Parkinson’s Disease. 2012;2012.
- Knapp M, Thorgrimsen L, Patel A, Spector A, Hallam A, Woods B, Orrell M. Cognitive stimulation therapy for people with dementia: cost-effectiveness analysis. The British Journal of Psychiatry. 2006 Jun;188(6):574-80.
- París AP, Saleta HG, de la Cruz Crespo Maraver M, Silvestre E, Freixa MG, Torrellas CP, Pont SA, Nadal MF, Garcia SA, Bartolomé MV, Fernández VL. Blind randomized controlled study of the efficacy of cognitive training in Parkinson’s disease. Movement Disorders. 2011 Jun;26(7):1251-8.
- Sammer G, Reuter I, Hullmann K, Kaps M, Vaitl D. Training of executive functions in Parkinson’s disease. Journal of the neurological sciences. 2006 Oct 25;248(1-2):115-9.
- Leung IH, Walton CC, Hallock H, Lewis SJ, Valenzuela M, Lampit A. Cognitive training in Parkinson disease A systematic review and meta-analysis. Neurology. 2015 Oct 30:10-212.
- Cerasa A, Gioia MC, Salsone M, Donzuso G, Chiriaco C, Realmuto S, Nicoletti A, Bellavia G, Banco A, D’amelio M, Zappia M. Neurofunctional correlates of attention rehabilitation in Parkinson’s disease: an explorative study. Neurological Sciences. 2014 Aug 1;35(8):1173-80.
- Peña J, Ibarretxe-Bilbao N, García-Gorostiaga I, Gomez-Beldarrain MA, Díez-Cirarda M, Ojeda N. Improving functional disability and cognition in Parkinson disease: randomized controlled trial. Neurology. 2014 Dec 2;83(23):2167-74.
- Petrelli A, Kaesberg S, Barbe MT, Timmermann L, Fink GR, Kessler J, Kalbe E. Effects of cognitive training in Parkinson’s disease: a randomized controlled trial. Parkinsonism & related disorders. 2014 Nov 1;20(11):1196-202.
- Zimmermann R, Gschwandtner U, Benz N, Hatz F, Schindler C, Taub E, Fuhr P. Cognitive training in Parkinson disease Cognition-specific vs nonspecific computer training. Neurology. 2014 Apr 8;82(14):1219-26.
- Dirnberger G, Jahanshahi M. Executive dysfunction in P arkinson’s disease: A review. Journal of neuropsychology. 2013 Sep;7(2):193-224.
- Edwards JD, Hauser RA, O’connor ML, Valdés EG, Zesiewicz TA, Uc EY. Randomized trial of cognitive speed of processing training in Parkinson disease. Neurology. 2013 Oct 8;81(15):1284-90.
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry research. 1991 Aug 1;38(2):143-62.
- Leroi I, McDonald K, Pantula H, Harbishettar V. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. Journal of geriatric psychiatry and neurology. 2012 Dec;25(4):208-14.
- Starkstein SE. Apathy in Parkinson’s disease: diagnostic and etiological dilemmas. Movement Disorders. 2012 Feb;27(2):174-8.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychological review. 1977 Mar;84(2):191.
- Choi J, Twamley EW. Cognitive rehabilitation therapies for Alzheimer’s disease: a review of methods to improve treatment engagement and self-efficacy. Neuropsychology review. 2013 Mar 1;23(1):48-62.
- Davis JD, Tremont G. Impact of frontal systems behavioral functioning in dementia on caregiver burden. The Journal of neuropsychiatry and clinical neurosciences. 2007 Jan;19(1):43-9.
- Chio A, Vignola A, Mastro E, Giudici AD, Iazzolino B, Calvo A, Moglia C, Montuschi A. Neurobehavioral symptoms in ALS are negatively related to caregivers’ burden and quality of life. European journal of neurology. 2010 Oct;17(10):1298-303.
- Ryan KA, Weldon A, Persad C, Heidebrink JL, Barbas N, Giordani B. Neuropsychiatric symptoms and executive functioning in patients with mild cognitive impairment: relationship to caregiver burden. Dementia and geriatric cognitive disorders. 2012;34(3-4):206-15.
- Ferrazzoli D, Ortelli P, Zivi I, Cian V, Urso E, Ghilardi MF, Maestri R, Frazzitta G. Efficacy of intensive multidisciplinary rehabilitation in Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2018 Jan 9:jnnp-2017.
- Tan SB, Williams AF, Kelly D. Effectiveness of multidisciplinary interventions to improve the quality of life for people with Parkinson’s disease: A systematic review. International journal of nursing studies. 2014 Jan 1;51(1):166-74.
- Knapp M, Thorgrimsen L, Patel A, Spector A, Hallam A, Woods B, Orrell M. Cognitive stimulation therapy for people with dementia: cost-effectiveness analysis. The British Journal of Psychiatry. 2006 Jun;188(6):574-80.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005 Apr;53(4):695-9.
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998 Feb 1;12(1):43-55.
- Wechsler D. WAIS-III, Wechsler adult intelligence scale: Administration and scoring manual. Psychological Corporation; 1997.
- Delis DC, Kaplan E, Kramer JH. D-KEFS: examiners manual. San Antonio, TX: The Psychological Corporation. 2001.
- Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Archives of clinical neuropsychology. 2004 Mar 1;19(2):203-14.
- Golden CJ, Freshwater SM. Stroop color and word test. 1978.
- Willis SL, Marsiske M. Manual for the everyday problems test. University Park: Pennsylvania State University. 1993:1-31.
- Shaw S, Oei TP, Sawang S. Psychometric validation of the Dysexecutive Questionnaire (DEX). Psychological assessment. 2015 Mar;27(1):138.
- Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio. 1996;78(2):490-8.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of internal medicine. 2006 May 22;166(10):1092-7.
- Jenkinson C, Fitzpatrick RA, Peto VI, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age and ageing. 1997 Sep 1;26(5):353-7.
- Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams‐Gray CH, Aarsland D. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Movement disorders. 2012 Mar;27(3):349-56.
- Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, Weintraub D. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009 Nov 24;73(21):1738-45.
- Bechara A. Iowa gambling task. Psychological Assessment Resources; 2007.
- Diener ED, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. Journal of personality assessment. 1985 Feb 1;49(1):71-5.
- Nilsson MH, Hagell P, Iwarsson S. Psychometric properties of the General Self‐Efficacy Scale in Parkinson’s disease. Acta Neurologica Scandinavica. 2015 Aug;132(2):89-96.
- Brennan L, Siderowf A, Rubright JD, Rick J, Dahodwala N, Duda JE, Hurtig H, Stern M, Xie SX, Rennert L, Karlawish J. The Penn Parkinson’s Daily Activities Questionnaire-15: Psychometric properties of a brief assessment of cognitive instrumental activities of daily living in Parkinson’s disease. Parkinsonism & related disorders. 2016 Apr 1;25:21-6.
- Roth RM, Gioia GA. Behavior rating inventory of executive function–adult version. Lutz, FL: Psychological Assessment Resources; 2005.
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. The gerontologist. 1980 Dec 1;20(6):649-55.
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. Journal of consulting and clinical psychology. 1991 Feb;59(1):12.
- Biundo R, Weis L, Fiorenzato E, Antonini A. Cognitive rehabilitation in Parkinson’s disease: is it feasible?. Archives of Clinical Neuropsychology. 2017 Nov 1;32(7):840-60.