Revista Iberoamericana de Neuropsicología
Vol. 2, No. 1: 30-42, enero-junio 2019.
Emotion regulation and neuropsychological status in functional neurological disorder variants
Bonnie M. Scott, M.S.1, Adriana M. Strutt, Ph.D., ABPP-CN2, Paula Lundberg-Love, Ph.D.3, Andrew L. Schmitt, Ph.D.4, Joshua Salzman1, Stephen K. Martin, Ph.D.5, Joseph Jankovic, M.D.2, Dawn Bowers, Ph.D., ABPP-CN1
1 Department of Clinical and Health Psychology, University of Florida
2 Department of Neurology, Baylor College of Medicine
3 Department of Psychology and Counseling, University of Texas at Tyler
4 University of Texas Health Science Center at Tyler
5 Martin Neurobehavioral Center, Tyler, TX
Corresponding author:
Bonnie M. Scott, M.S.
University of Florida
PO Box 100165
Gainesville, FL 32610-0165
Phone: 713-724-8142
Email: bonnie.m.scott@phhp.ufl.edu
Emotion regulation and neuropsychological status in functional neurological disorder variants
Few clinically meaningful treatment options exist for patients with functional neurological disorders (FND) due to limited understanding of within-group differences in cognitive and emotional factors that may differentially influence mental health outcomes. This study aimed to determine the relationship between emotion regulation strategies (suppression vs. reappraisal), psychological symptoms, and cognitive status in two FND variants: non-epileptic seizures (NES) and other functional (hyperkinetic) movement disorders (FMD). Thirty-two patients (NES = 16; FMD = 16) completed a neuropsychological battery including self-report questionnaires of emotion regulation and psychopathology. In the NES group, lower cognition was associated with more severe PTSD symptoms, greater suppression and lower positive emotions. In the FMD group, lower cognition was associated with more severe PTSD symptoms and greater reappraisal. When controlling for general cognition, individuals classified as “suppressors” had more trauma events and symptoms of dissociation, greater internalizing dysfunction, and more severe emotional distress than individuals classified as “re-appraisers.” Results suggest individual differences in cognitive function and habitual behavioral tendencies such as emotion regulation may be important considerations in tailoring treatment of posttraumatic distress for FND variants. Current findings also suggest that future clinical trials considering FND variants separately may facilitate the development of symptom-specific treatment approaches.
Key Words: Functional neurological disorders; Psychogenic; Conversion; Cognition; Executive function
Functional neurological disorders (FND) include a myriad of sensorimotor symptoms with unknown neurologic etiology. Until recently, this group of disorders was also referred to as “psychogenic” disorders and both terms are currently used interchangeably1. These symptoms are thought to reflect a conversion disorder variant2. The most common symptom phenotypes in a population of patients with FND presenting as functional (psychogenic) movement disorders (FMD) are tremor (40%), dystonia (31%), and myoclonus (13%), but other movement disorders such as parkinsonism, tics, stereotypies hemifacial spasm, and oculomotor and speech abnormalities can also occur3,4. Another FND variant, more commonly seen in epilepsy centers are individuals with non-epileptic seizures (NES). These individuals have paroxysmal episodes resembling epilepsy—most commonly imitating epileptic complex partial episodes—but which occur in the absence of electroencephalographic (EEG) abnormalities5,6.
Traditionally, FND has been viewed as the physical manifestation of traumatically induced psychological distress, as trauma and stress-related symptoms are frequently observed in these patients1,7. The significantly higher prevalence of posttraumatic stress disorder (PTSD) and associated symptoms of dissociation in this patient population have led some researchers to suggest that FND may be a related condition7,8. The dissociative symptoms commonly seen in these patients have been shown to correlate significantly with both physical and sexual abuse9, as well as other forms of childhood trauma10. However, not all individuals suffering from FND report a history of abuse or trauma, and there remains a paucity of research investigating variables that may potentially mediate the relationship between psychological distress and the development of FNDs. While this void may have contributed to the conflicting findings regarding FNDs, such results could also be the product of inappropriate methodology, the most notable of which includes the heterogeneity of FND samples utilized in research, along with a tendency for investigators to neglect phenotype distinctions.
The few studies that have compared the features of FND patients with different motor manifestations have yielded significant results. For instance, a recent study conducted by Hopp et al.11 suggested different demographic profiles and clinical manifestations (e.g., altered consciousness, episodic symptoms, and lateralization) may characterize patients with other FMD phenotypes as compared to those with NES. Additional differences observed have included a higher rate of reported trauma and environmental stressors, as well as greater borderline personality features and external control orientation associated with NES patients than those with other FMD phenotypes12,13. There is also now preliminary data suggesting various facets of emotional processing (e.g., attributional style, coping strategies, etc.) may mediate the relationship between early life experiences and subsequent development of these sensorimotor disturbances14-16. Although such findings have yet to be replicated, this recent trend in empirical investigations with FND patients suggests that distinguishing symptom-specific subgroups with potentially divergent neurobiological underpinnings may improve our understanding of these sensorimotor disturbances and thus be more useful in tailoring treatment.
The overall goal of the current study was to advance our conceptualization of FND by examining the relationship between emotion regulation strategies, psychological symptoms, and cognitive status in two FND variants: NES and FMD. We had two specific aims: (1) To determine whether cognitive dysfunction is associated with emotion regulation strategies and psychological symptoms in FND; and (2) To identify potential emotional and neurocognitive differences between NES and FMD. Our working hypothesis was that the cognitive profile of the combined sample would be characterized by frontal-executive inefficiencies (i.e., working memory, inhibition, set-shifting), and that lower cognitive function would be associated with greater emotional distress. This hypothesis is based on two lines of evidence: (1) neuroimaging findings that have implicated functional abnormalities in frontal and limbic regions in FND patients; and (2) observations that emotion regulation efficacy is predicated upon intact cognitive control mechanisms17-19. Based on the view that frontal-executive control is necessary for effective emotion regulation20, we also hypothesized that NES patients would demonstrate worse cognitive dysfunction than FMD patients given previous observations of more severe psychopathology in patients with NES13.
Participants
Data collection was conducted at Baylor College of Medicine (BCM) and St. Luke’s Episcopal Hospital in Houston, Texas, as well as Martin Neurobehavioral Center (MNC) in Tyler, Texas. Potential participants were identified based on previous neurological evaluations and appropriate medical assessments establishing an FND diagnosis. For NES patients, this included continuous video-EEG monitoring showing no indications of epileptiform activity, and for FMD patients, adherence to Fahn and Williams diagnostic criteria (i.e., inconsistency/ incongruency, other false neurological signs, distractibility, multiple somatizations, psychiatric disturbance)21. Other inclusion criteria were male and female English-speaking adults ages eighteen to sixty-five. The following exclusion criteria were employed: 1) presence of an underlying neurological disorder; 2) current or past psychotic symptoms that could interfere with assessment; 3) substance abuse disorder within the past six months; 4) traumatic brain injury; 5) unstable medical condition or clinically significant abnormal laboratory results; and 6) mixed etiologies (e.g., concurrent epilepsy and NES). Altogether, 84 patients with an FND diagnosis were identified as potential participants and screened for possible inclusion. Of those patients screened, twenty-seven were excluded due to age (9), poor English mastery (2), traumatic brain injury (3), cerebrovascular accident (4), multiple sclerosis (1), and other comorbid neurological conditions (8). Eighteen patients declined to participate, and seven others were scheduled to participate but did not complete the evaluation.
The final sample consisted of thirty-two patients who ranged in age from 18-64 years (M = 42.2, SD = 12.3) with 10-20 years of education (M = 13.6, SD = 2.3). Each patient’s clinical presentation was characterized based on symptoms documented by the neurologist in their electronic medical chart. This resulted in the following symptom classifications: 16 NES (50.0%), 2 dystonia (6.3%), 1 bilateral tremor (3.1%), 1 left-sided tremor (3.1%), 3 right-sided tremor (9.4%), 1 myoclonus (3.1%), 2 gait disturbance (6.3%), 2 bilateral tremor and gait disturbance (6.3%), 1 tic and stereotypies (3.1%), and 3 mixed facial symptoms (e.g., dystonia, tics, orofacial dyskinesia, and blepharospasm; 9.4%). Because the research protocol used for the present study is not a routine component of standard clinical care for individuals with these diagnoses, it was offered as a free service with no incentive. This study was approved by BCM’s IRB and all participants provided informed consent.
Procedure
Each patient participated in a clinical interview eliciting information about any interim changes in their medical history, including psychiatric, psychosocial, and potential trauma history, as well as the circumstances surrounding the onset and course of their motor symptoms. Participants were administered a brief neuropsychological battery consisting of standardized measures to assess frontal-executive functions, determine the validity of test performance, and provide an estimate of premorbid intellect and general cognitive status. These included: a) measures of effort (Test of Memory Malingering [TOMM]22, Rey Fifteen Item Memory Test [Rey-15]23,24); b) a cognitive screener (Montreal Cognitive Assessment [MoCA]25); c) an estimate of premorbid intellect (Wechsler Test of Adult Reading [WTAR])26; d) auditory attention span (Digit Span subtest of the Wechsler Adult Intelligence Scale, Fourth Edition [WAIS-IV])27; and e) executive function tasks associated with problem solving (Wisconsin Card Sorting Test, 64 Card Version [WCST-64])28; verbal fluency (Letter Fluency [FAS] and Semantic Fluency [Animals])29,30; and speeded set-shifting (Trail Making Test, Part A [TMT-A] and B [TMT-B])31.
Participants completed six standard self-report questionnaires to assess mood and trauma-related symptoms, use of emotion regulation strategies, and personality/behavioral tendencies previously associated with FND6. These included: the Beck Depression Inventory, Second Edition (BDI-II)32; Penn State Worry Questionnaire (PSWQ)33; the Posttraumatic Stress Disorder Checklist – Civilian version (PCL-C)34; Dissociative Experiences Scale, Second Edition (DESII)35; Emotion Regulation Questionnaire (ERQ); and the Minnesota Multiphasic Personality Inventory, Second Edition, Restructured Format (MMPI-2-RF)36. All instruments were administered and scored according to standardized procedures. Additional data regarding each participant’s current condition, treatment, and medical history were later obtained via review of medical records.
Raw scores were used for measures involving raw score cutoffs (i.e., MoCA < 26, TOMM: Trial 2 < 45, Rey15 < 9, BDI-II ≥ 14, PSWQ ≥ 40, PCL ≥ 30, ERQ: Dominant > Non-dominant strategy, and DES-II ≥ 30). Age-adjusted standard scores were used for the WTAR26 and demographically adjusted T-scores were used for the MMPI-2-RF36, WAIS-IV Digit Span27, WCST-6428, TMT – Parts A and B, and Verbal Fluency measures29.
Statistical analyses
Statistical analyses were conducted via IBM® SPSS version 18.0 for Windows. Pearson’s correlations were used to examine relationships between variables, while multi-dimensional chi-square tests were used to compare categorical variables between groups, including demographic variables and classifications of performance on the outcome measures. Due to a limited sample, each continuous variable was carefully screened for potential violations of assumptions underlying parametric procedures. Standard statistical transformations as outlined by Tabachnick & Fidell37 were applied to those variables violating the assumption of normality. Mann-Whitney tests were used to examine between-group differences for variables that failed to achieve normalization with statistical transformations, while independent sample t-tests were used to compare all other continuous variables between groups.
As age and education are demographic variables known to significantly influence performance on neuropsychological tests, these two variables were screened for their potential utility as covariates for group comparisons on neurocognitive measures not already corrected for both of these demographic variables (i.e., WTAR and WAIS-IV Digit Span). When examined as covariates, age was not significantly related to either outcome measure, and education was only significantly related to WTAR scores. Analysis of covariance (ANCOVA) with education as a covariate was used to examine between-group differences on WTAR scores, while all other neurocognitive measures were assessed via independent sample t-tests and Mann-Whitney tests, as outlined above. Additionally, linear regression analyses were used to examine potential predictors of cognition for the total sample. The stepwise method was used in these analyses due to the exploratory nature of the present study. Finally, a binary logistic regression analysis was employed to identify potential predictors of group membership (i.e., NES versus FMD).
Demographic comparisons
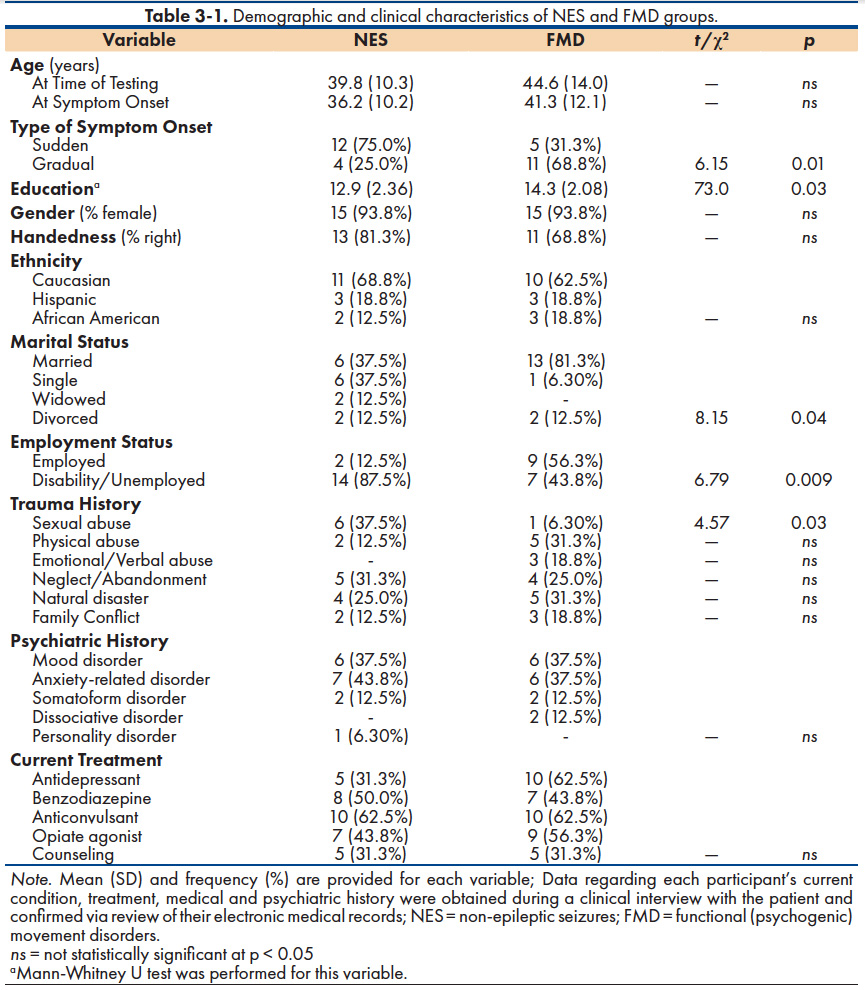
A comparison of NES and FMD groups along demographic variables is provided in Table 3-1. As shown, FMD participants were more educated and more likely to be married and employed at the time of testing than their NES counterparts. A significantly greater proportion of NES patients (75.0%) reported a sudden onset of their motor symptoms, whereas gradual symptom onset characterized the majority of FMD participants (68.8%). A history of some form of traumatic experience was reported by the majority of participants in both the NES (81.3%) and FMD (75.0%) groups. However, the only significant between-group difference in trauma history was self-reported sexual abuse (NES = 37.5%; FMD = 6.8%).
Neuropsychological results
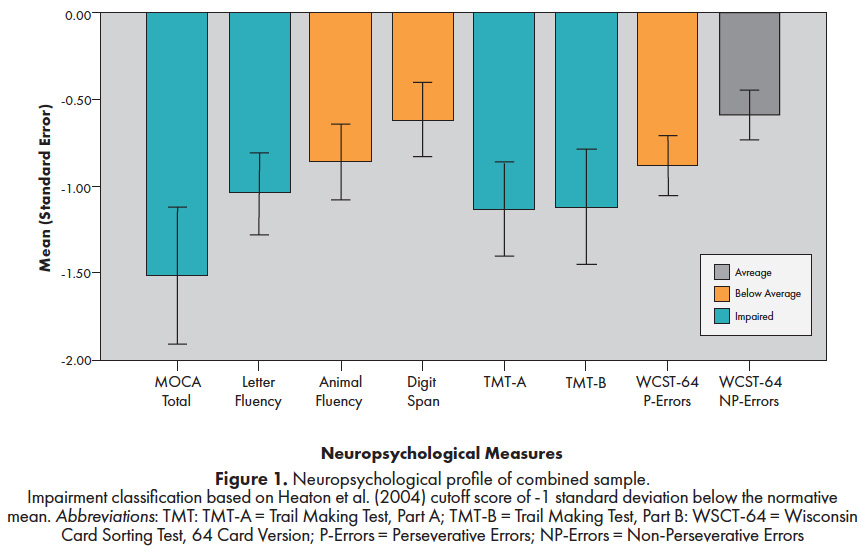
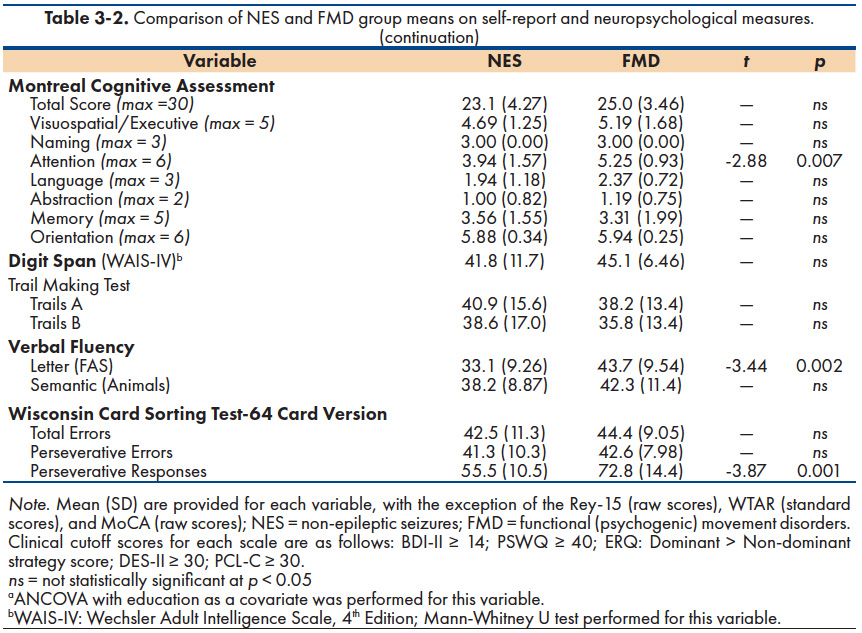
The neuropsychological profile of the combined groups in relation to demographically-corrected normative data is provided in Figure 1. As a group, performance on a general cognitive screening measure (MoCA) and two measures of executive function (Letter fluency and Trail Making Test, Part B), fell more than 1.0 standard deviation below the mean29. Performance on three other measures (Animal Fluency, Digit Span, and WCST-64 Perseverative Errors) ranged from -1.0 to -0.5 standard deviations below the mean. Overall, mean estimated IQ scores were in the average range (WTAR: NES = 95; FMD = 101) and all participants scored within normal limits on symptom validity measures (TOMM and Rey-15). As shown in Table 3-2, the two FND groups scored similarly across neurocognitive measures except for statistically worse performance by NES patients on the following: MoCA Attention subscale score, Letter Fluency, and WCST-64 Perseverative Responses.
Mood and emotion symptom findings
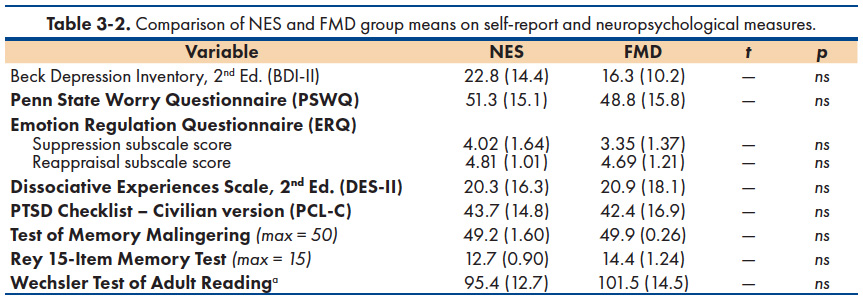
The majority of participants scored above clinical cutoff on three self-report measures: depression symptoms (BDI-II ≥ 14 = 56.8%), worry/rumination (PSWQ ≥ 40 = 72%) and PTSD symptoms (PCL-C ≥ 30 = 76%). Less than 1/3 of the combined sample scored above the clinical cutoff on a measure of dissociation symptoms (DES-II = 29.2%). However, the two FND subgroups did not differ significantly in their mean scores on measures of depression (BDI-II), worry/rumination (PSWQ), posttraumatic symptoms (PCL), dissociation (DES-II), or use of suppression and reappraisal strategies for regulating emotion (ERQ). Table 3-2 shows the mean scores of the two groups across these psychological measures.
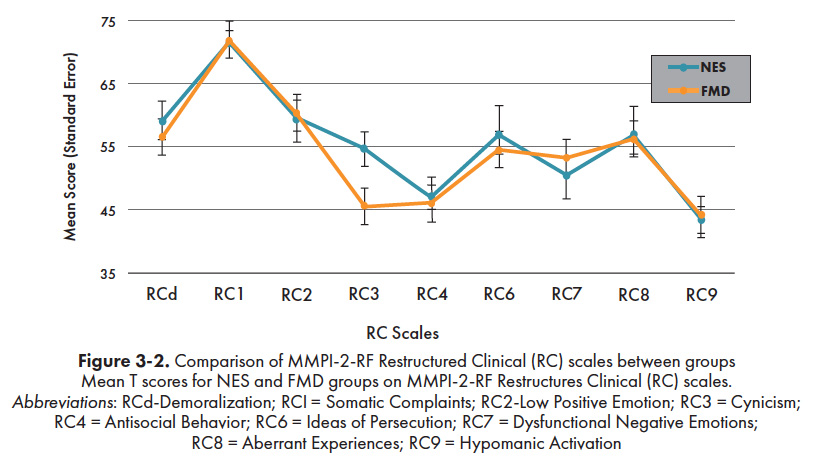
Figure 3-2 depicts the profile scores of the NES and FMD subgroups across the 9 clinical scales (i.e., Restructured Clinical [RC] Scales) of the MMPI-2-RF. Using the traditional clinical cutoff of T ≥ 65, the only clinically significant elevation for both groups was on the Somatic Concerns scale (RC Scale 1). Group means on all other RC Scales were within normal limits. The two groups did not differ significantly in their mean RC Scale scores, with the exception of significantly higher mean scores on the Cynicism scale (RC Scale 3) by the NES group (M = 54.7) than the FMD group [M = 45.6; t(24) = 2.34, p = 0.03]. A significantly higher proportion of FMD participants had elevated scores on the Negative Emotionality/Neuroticism scale [NES = 6.30%, FMD = 43.8%; χ2(1) = 4.21, p = 0.04] of the Revised Personality Psychopathology Five (PSY-5) Scales, while a significantly greater number of NES patients obtained clinically elevated scores on the Suicidal Ideation scale [NES = 18.8%, FMD = 0.0%; χ2(1) = 5.11, p = 0.02] of the Internalizing Scales.
Predictors of cognition
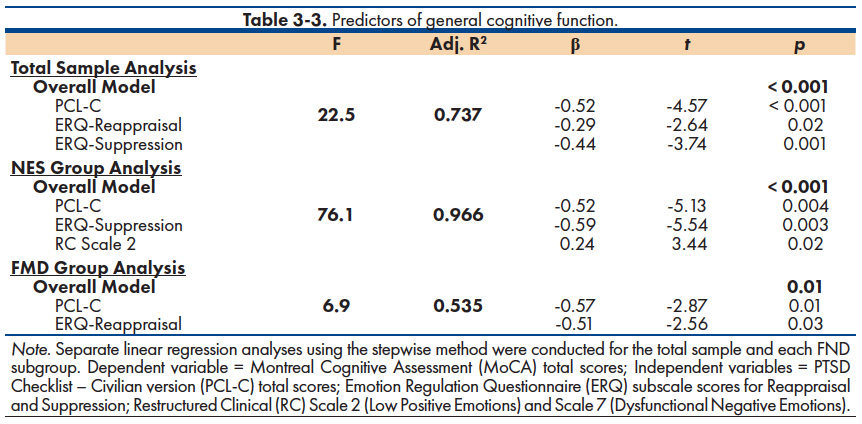
A series of stepwise linear regression analyses were used to determine if emotional distress was associated with cognitive function in general and with executive function specifically. The MoCA total scores were used as an index of general cognition. An Executive Function Composite score was computed by taking the average of the mean T-scores of two frontal lobe mediated tasks, Letter Fluency and WCST-64 Perseverative Responses, both of which differed based on group membership. Given the limited sample, we restricted our selection of independent variables to those emotion measures with a strong theoretical basis for inclusion: 1) trauma symptoms (PCL); 2) emotion regulation strategies (ERQ); and 3) dissociable facets of emotional distress from the MMPI-2-RF, represented by Low Positive Emotions [RC Scale 2] and Dysfunctional Negative Emotions [RC Scale 7].
Table 3-3 shows the results of these linear regression analyses predicting general cognitive function. As shown, the overall model was significant [F(3, 28) = 22.5, p < 0.001, Adjusted R2 = 0.737] for the total sample, such that lower MoCA scores were associated with higher PTSD symptoms (PCL-C) and higher ERQ-Reappraisal and Suppression scores. However, slightly different findings emerged when the two groups were examined separately. For the NES group, lower MoCA scores were associated with higher PTSD symptoms (PCL-C), greater use of emotional suppression (ERQ-Suppression scores), and lower positive emotions (RC Scale 2). This combination of variables accounted for 99.6% of the variance in general cognitive function in the NES group. For the FMD group, lower MoCA scores were associated with higher PTSD symptoms (PCL) and greater use of emotion reappraisal (ERQ-Reappraisal scores), with this combination of variables accounting for 53.5% of the variance in general cognitive function.
With respect to executive function, a significant model was found for the total sample [F(1, 30) = 6.34, p = 0.02, Adjusted R2 = 0.224]. Lower Executive Function scores were associated with higher endorsement of emotional suppression strategies (ERQ-Suppression: β = -0.47, t = -2.52, p = 0.02). No other emotion scores were associated with MoCA performance. There were no significant findings when similar regression analyses were conducted separately for each group.
Exploratory analyses
Psychological Differences Based on Emotion Regulation Strategies. Given that emotion regulation strategies, particularly greater suppression, were associated with worse MoCA performance, we examined the relationship between emotion regulation and other psychological symptoms (i.e., PTSD, etc.). Participants were classified as “reappraisers” or “suppressors” based on their highest mean ERQ subscale score. Using this metric, 56% of the participants were classified as reappraisers and 44% were classified as suppressors. After controlling for MoCA performance, analyses of covariance indicated that individuals in the suppressor group reported greater psychological distress than those in the appraiser group. Thus, the suppressor group had significantly more trauma events [F(1, 30) = 12.5, p = 0.002], more interpersonal and internalizing dysfunction (Family Problems: p = 0.003; Social Avoidance: p = 0.01; Suicidal Ideation: p = 0.009; Self-Doubt: p < 0.001), more severe emotional distress (BDI-II: p = 0.008; PSWQ: p = 0.004), and more symptoms of dissociation (DES-II: p = 0.004) than the reappraisers.
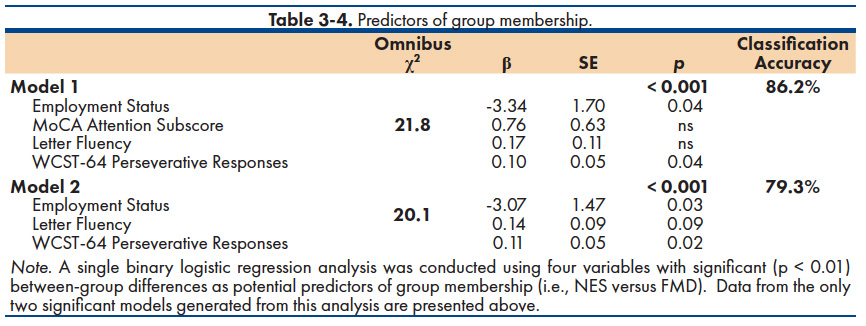
Predicting Subgroup Membership. We also examined possible predictors of group membership (NES versus FMD). Given our small sample, a conservative analytic approach was adopted—using four variables with significant (p < 0.01) between-group differences: Employment status, MoCA Attention subscale score, WCST-64 Perseverative Responses, and Letter Fluency. Based on this criterion, a single binary logistic regression analysis was conducted and two significant models were generated (Table 3-4). The first model included all four predictor variables, yielding a classification accuracy of 86.2% (NES = 87.5%; FMD = 84.6%). Separately, however, only employment status and WCST-64 Perseverative Responses were statistically significant predictors (p < 0.05). A second model was generated consisting of functional status, WCST-64 Perseverative Responses, and a trend for Letter Fluency. This combination of variables resulted in 79.3% of the total sample being correctly classified (NES = 75.0%; FMD = 84.6%). These findings are considered preliminary, however, and must be interpreted with caution given the limited sample.
There were three major findings. First, consistent with previous observations, FMD participants endorsed a higher prevalence of clinically significant post-traumatic symptoms and other emotion/ mood symptoms relative to the general population7. Second, as a group, both NES and FMD patients demonstrated a general cognitive inefficiency (i.e., reduced total MoCA score) as well as executive weaknesses. The latter was based on mild reductions on tasks of letter fluency and set-shifting. These frontal-executive weaknesses occurred in the context of average premorbid intellect and valid effort during the neuropsychological assessment. Although the disruptive effects of psychological distress may underlie these frontal-executive and cognitive inefficiencies, we found that greater use of emotion regulation strategies involving “suppression” (rather than re-appraisal) was associated with worse executive function. In terms of overall cognitive status, both posttraumatic distress and the habitual use of either suppression or reappraisal was associated with lower overall cognitive function. Findings are consistent with previous neuroimaging studies documenting functional abnormalities in neural regions involved in emotion regulation (e.g., anterior cingulate, ventromedial PFC, amygdala), cognitive control and motor inhibition (e.g., anterior cingulate, dorsolateral PFC, inferior frontal gyrus) in FMD patients38.
The third major finding pertained to differences between the two FND subgroups. While FMD patients generally outperformed their NES counterparts across neuropsychological measures, frank statistical differences were found on measures of simple auditory attention, letter fluency and perseverative responding, with the NES group performing worse than the FMD group. On mood and emotion measures, NES and FMD participants reported similar rates of self-reported trauma, post-traumatic symptoms, worry and depressive symptoms. However, consistent with previous research12,13, NES patients reported a significantly higher occurrence of sexual abuse than FMD patients (i.e., 6 to 1), were less educated (by 2 years), and less frequently married and employed at the time of testing. The three factors that distinguished the NES and FMD groups were employment status, letter fluency and perseverative response tendencies. Based on these findings, we conclude that executive inefficiencies are more evident in patients with NES than FMD. This may result in diminished capacity to navigate social interactions and appropriately modify behavioral strategies according to environmental feedback by patients with NES than those with FMD.
Alternatively, the unexpected association observed between cognition, traumatic stress and different emotion regulation strategies in NES vs. FMD groups may refine our conceptualization of FND. Specifically, while findings support the traditionally proposed role of trauma in FND, they39 further implicate individual differences in emotion regulation strategies as important variables that may mediate the relationship between early adverse experiences and mental health outcomes. Consistent with previous research by Gross and John40, the habitual use of emotional suppression was associated with greater negative emotional experience (depression and worry/rumination) and interpersonal dysfunction (family problems and social avoidance). However, there was an exponentially higher number of traumatic events and dissociative symptoms found among habitual suppressors vs. reappraisers. Results suggest that victims of multiple traumatic experiences may rely on suppression strategies to manage their distressing emotions, and perhaps more likely to experience dissociative symptoms of posttraumatic stress. These findings complement the results of previous investigations suggesting various facets of emotional processing may mediate the relationship between early life experiences and subsequent development of these sensorimotor disturbances14-16.
Overall, the results of this study add to a growing body of literature suggesting that NES and FMD may represent phenotypic variants of similar underlying conditions. To date, the majority of research investigating FND subgroup differences has been limited to either a single phenotypic variant or a single functional domain of interest (e.g., psychological distress or neurocognitive function). In contrast, the present study included a heterogeneous sample of hyperkinetic FND (NES and FMD) who were examined across multiple domains, including psychologic-emotion, psychosocial and neurocognitive. Although such findings are considered preliminary, results of the current study will provide a foundation for future investigations of FND variants that may facilitate the development of symptom-specific treatment approaches. For instance, the greater emotional suppression tendencies and suicidal ideation in NES patients may be targeted with adaptive emotion regulation training and perhaps off-label use of pharmacological agents with demonstrated efficacy in reducing suicidal ideation. Alternatively, the greater negative emotionality found in FMD patients has been associated with general deficits in attentional control including difficulty disengaging attentional resources from negatively valanced stimuli41. Thus, patients with similar functional neurological symptoms may benefit from therapeutic interventions incorporating biofeedback training, in which patients learn to restructure targeted patterns of brainwaves through the provision of information on their cortical electrical activity42,43.
Limitations & future directions
The current findings should be interpreted with caution due to the small sample and the high number of statistical comparisons. Moreover, our limited sample precluded further subdivision of the FMD group into more homogeneous motor subgroups (e.g., tremor versus gait disturbances, etc.). Given that these participants were seen at specialized tertiary care centers, the sample may have included more severe FND cases. Future investigations should strive to investigate a larger sample, provide comparisons between more homogeneous motor subgroups, and examine the frequency and type of previous therapeutic interventions. Future research efforts should also be directed toward furthering our understanding of important biological and psychosocial differences between FND patients that may facilitate the development of more effective and individualized therapeutic interventions.
Closing remarks
In sum, the current study adds to the literature by highlighting (1) the clinical utility of examining FND patients with different sensorimotor symptoms separately and (2) the importance of investigating reciprocal relationships between psychological symptoms, cognitive functioning, and habitual behavioral tendencies (i.e., emotion regulation strategies) that may mediate the relationship between early adverse experiences and mental health outcomes. Although the causative mechanisms underlying the development of FND have yet to be determined and are probably multi-factorial, our findings suggest that both cognitive weaknesses and habitual behavioral tendencies may play an important role in the clinical presentation of patients with FND.
Financial Disclosure/Conflict of Interest
This work was supported by NINDS T32-NS082168 and NIH TL1TR001428 & UL1TR001427.
- Jankovic J. “Psychogenic” versus “functional” movement disorders? That is the question. Movement Disorders. 2014;29(13):1697-8.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub; 2013.
- Thenganatt MA, Jankovic J. Psychogenic tremor: a video guide to its distinguishing features. Tremor and Other Hyperkinetic Movements. 2014;4.
- Thenganatt MA, Jankovic J. Psychogenic (functional) parkinsonism. Handbook of Clinical Neurology. 2016;139(3):259-262.
- Alsaadi TM, Marquez AV. Psychogenic nonepileptic seizures. Am Fam Physician. 2005;72(5):849-56.
- Perez DL, LaFrance WC. Nonepileptic seizures: an updated review. CNS spectrums. 2016;21(3):239-46.
- Fiszman A, Alves-Leon SV, Nunes RG, Isabella DA, Figueira I. Traumatic events and posttraumatic stress disorder in patients with psychogenic nonepileptic seizures: a critical review. Epilepsy & Behavior. 2004;5(6):818-25.
- Dikel TN, Fennell EB, Gilmore RL. Posttraumatic stress disorder, dissociation, and sexual abuse history in epileptic and nonepileptic seizure patients. Epilepsy & Behavior. 2003;4(6):644-50.
- Draijer N, Langeland W. Childhood trauma and perceived parental dysfunction in the etiology of dissociative symptoms in psychiatric inpatients. American Journal of Psychiatry. 1999;156(3):379-85.
- Roelofs K, Keijsers GP, Hoogduin KA, Näring GW, Moene FC. Childhood abuse in patients with conversion disorder. American Journal of Psychiatry. 2002;159(11):1908-13.
- Hopp JL, Anderson KE, Krumholz A, Gruber-Baldini AL, Shulman LM. Psychogenic seizures and psychogenic movement disorders: are they the same patients?. Epilepsy & Behavior. 2012;25(4):666-9.
- Duncan R, Oto M. Predictors of antecedent factors in psychogenic nonepileptic attacks Multivariate analysis. Neurology. 2008;71(13):1000-5.
- Stone J, Sharpe M, Binzer M. Motor conversion symptoms and pseudoseizures: a comparison of clinical characteristics. Psychosomatics. 2004;45(6):492-9.
- Gul A, Ahmad H. Cognitive deficits and emotion regulation strategies in patients with psychogenic nonepileptic seizures: a task-switching study. Epilepsy & Behavior. 2014;32:108-13.
- Myers L, Fleming M, Lancman M, Perrine K, Lancman M. Stress coping strategies in patients with psychogenic non-epileptic seizures and how they relate to trauma symptoms, alexithymia, anger and mood. Seizure. 2013;22(8):634-9.
- Teodoro T, Edwards MJ. Functional movement disorders. Current opinion in neurology. 2016;29(4):519-25.
- van der Kruijs SJ, Bodde NM, Vaessen MJ, Lazeron RH, Vonck K, Boon P, Hofman PA, Backes WH, Aldenkamp AP, Jansen JF. Functional connectivity of dissociation in patients with psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry. 2012;83(3):239-47.
- Voon V, Brezing C, Gallea C, Ameli R, Roelofs K, LaFrance Jr WC, Hallett M. Emotional stimuli and motor conversion disorder. Brain. 2010;133(5):1526-36.
- Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology. 2010;74(3):223-8.
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage. 2004;23(2):483-99.
- Fahn S, Williams DT. Psychogenic dystonia. Advances in neurology. 1988;50:431-55.
- Tombaugh TN. Test of memory malingering: TOMM. New York/Toronto: MHS; 1996.
- Rey A. L’examen Clinique en psychologie. Paris: Presses Universitaires de France; 1964.
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment (4th ed.). New York, NY: Oxford University Press; 2004.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695-9.
- Wechsler D. Wechsler Test of Adult Reading: WTAR. San Antonio, TX: The Psychological Corporation; 2001.
- Wechsler, D. Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV): Administration and scoring manual. San Antonio, TX: The Psychological Corporation; 2008.
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test®-64 Card Version (WCST-64): Professional manual. Lutz, FL: Psychological Assessment Resources; 2000.
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources; 2004.
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985.
- Reitan RM. Trail making test: Manual for administration and scoring. Tucson, AZ: Reitan Neuropsychological Laboratory; 1992.
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II, San Antonio, TX: Psychological Corporation; 1996.
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the penn state worry questionnaire. Behaviour research and therapy. 1990;28(6):487-95.
- Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Inannual convention of the international society for traumatic stress studies, San Antonio, TX. 1993;24(462).
- Stockdale GD, Gridley BE, Balogh DW, Holtgraves T. Confirmatory factor analysis of singleand multiple-factor competing models of the dissociative experiences scale in a nonclinical sample. Assessment. 2002;9(1):94-106.
- Tellegen A, Ben-Porath YS. MMPI-2-RF: Technical Manual. Minneapolis, MN: University of Minnesota Press; 2008.
- Tabachnick BG, Fidell LS. Using Multivariate Statistics (4th ed). New York, NY: HarperCollins; 2001.
- Aybek S, Vuilleumier P. Imaging studies of functional neurologic disorders. Handbook of clinical neurology. 2016;139(1):73-84.
- Kanaan RA, Duncan R, Goldstein LH, Jankovic J, Cavanna AE. Are psychogenic non-epileptic seizures just another symptom of conversion disorder?. J Neurol Neurosurg Psychiatry. 2017;0:1-5
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85(2):348-362.
- Bredemeier K, Berenbaum H, Most SB, Simons DJ. Links between neuroticism, emotional distress, and disengaging attention: Evidence from a single-target RSVP task. Cognition and Emotion. 2011;25:1510-1519.
- Choi S, Chi S, Chung S, Kim J, Ahn C., Kim H. Is alpha wave neurofeedback effective with randomized clinical trials in depression? A pilot study. Neuropsychobiology. 2010;63(1):43-51.
- Sacchet MD, Hamilton JP, Glover GH, Bagarinao E, Chang C, Mackey S, Gotlib IH. The effects of neurofeedback training of salience network nodes on affective biases in major depression. Biol Psychiatry. 2013;73(9):220S.